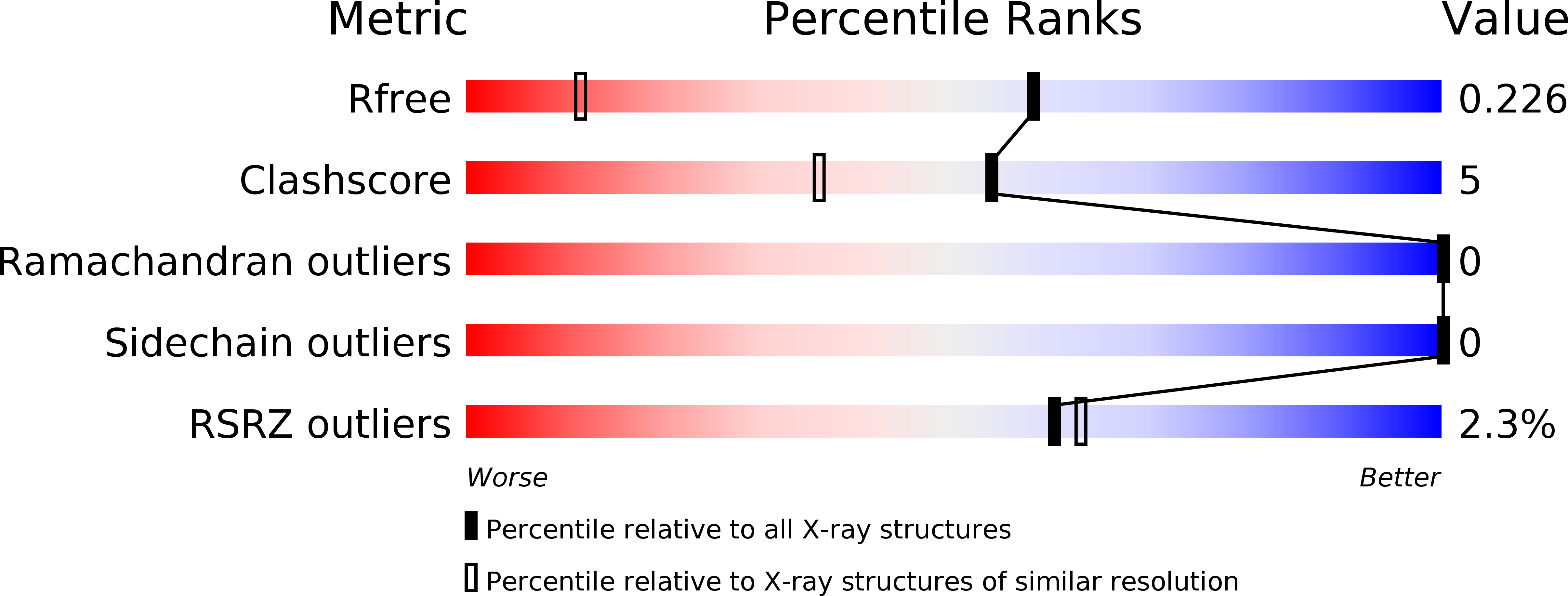
Structure and stability of the C-terminal helical bundle of the E. coli mechanosensitive channel of large conductance.
Walton, T.A., Rees, D.C.(2013) Protein Sci 22: 1592-1601
- PubMed: 24038743
- DOI: https://doi.org/10.1002/pro.2360
- Primary Citation of Related Structures:
4LKU - PubMed Abstract:
The crystal structure of the cytoplasmic domain (CTD) from the mechanosensitive channel of large conductance (MscL) in E. coli has been determined at 1.45 Å resolution. This domain forms a pentameric coiled coil similar to that observed in the structure of MscL from M. tuberculosis and also found in the cartilage oligomeric matrix protein (COMPcc). It contains canonical hydrophobic and atypical ionic interactions compared to previously characterized coiled coil structures. Thermodynamic analysis indicates that while the free EcMscL-CTD is less stable than other coiled coils, it is likely to remain folded in context of the full-length channel.
Organizational Affiliation:
Division of Chemistry and Chemical Engineering, Howard Hughes Medical Institute, California Institute of Technology, Pasadena, California, 91125.