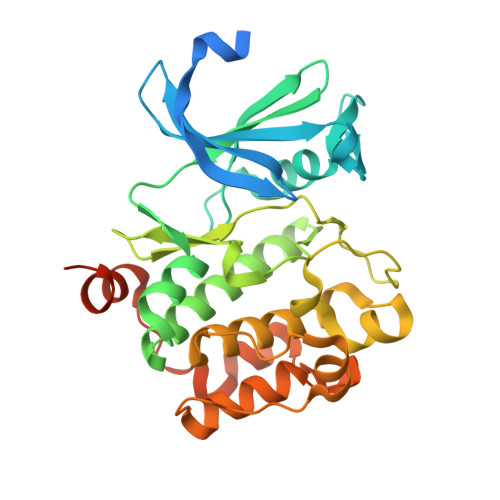
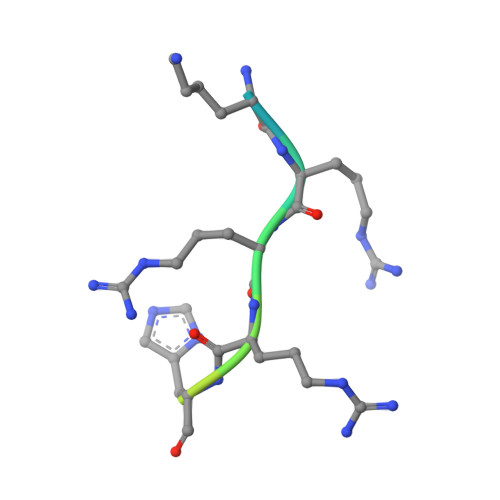
Crystal structure of pim1 kinase in complex with a pyrido[4,3-d]pyrimidine derivative suggests a unique binding mode.
Lee, S.J., Han, B.G., Cho, J.W., Choi, J.S., Lee, J., Song, H.J., Koh, J.S., Lee, B.I.(2013) PLoS One 8: e70358-e70358
- PubMed: 23936194
- DOI: https://doi.org/10.1371/journal.pone.0070358
- Primary Citation of Related Structures:
4JX3, 4JX7 - PubMed Abstract:
Human Pim1 kinase is a serine/threonine protein kinase that plays important biological roles in cell survival, apoptosis, proliferation, and differentiation. Moreover, Pim1 is up-regulated in various hematopoietic malignancies and solid tumors. Thus, Pim1 is an attractive target for cancer therapeutics, and there has been growing interest in developing small molecule inhibitors for Pim1. Here, we describe the crystal structure of Pim1 in complex with a newly developed pyrido[4,3-d]pyrimidine-derivative inhibitor (SKI-O-068). Our inhibitor exhibits a half maximum inhibitory concentration (IC50) of 123 (±14) nM and has an unusual binding mode in complex with Pim1 kinase. The interactions between SKI-O-068 and the Pim1 active site pocket residue are different from those of other scaffold inhibitor-bound structures. The binding mode analysis suggests that the SKI-O-068 inhibitor can be improved by introducing functional groups that facilitate direct interaction with Lys67, which aid in the design of an optimized inhibitor.
Organizational Affiliation:
Biomolecular Function Research Branch, Research Institute, National Cancer Center, Goyang, Gyeonggi, Republic of Korea.