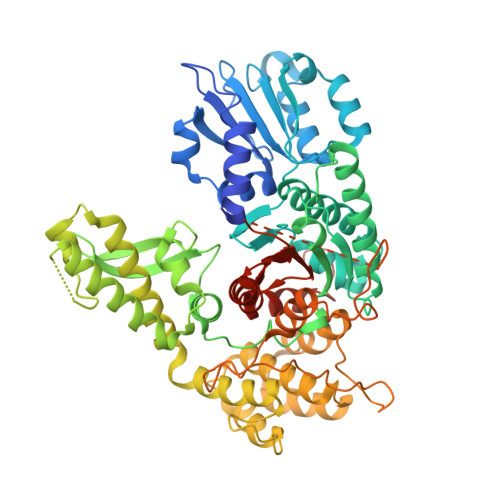
Syntaxin1a variants lacking an N-peptide or bearing the LE mutation bind to Munc18a in a closed conformation.
Colbert, K.N., Hattendorf, D.A., Weiss, T.M., Burkhardt, P., Fasshauer, D., Weis, W.I.(2013) Proc Natl Acad Sci U S A 110: 12637-12642
- PubMed: 23858467
- DOI: https://doi.org/10.1073/pnas.1303753110
- Primary Citation of Related Structures:
4JEH, 4JEU - PubMed Abstract:
In neurons, soluble N-ethylmaleimide-sensitive factor attachment receptor (SNARE) proteins drive the fusion of synaptic vesicles to the plasma membrane through the formation of a four-helix SNARE complex. Members of the Sec1/Munc18 protein family regulate membrane fusion through interactions with the syntaxin family of SNARE proteins. The neuronal protein Munc18a interacts with a closed conformation of the SNARE protein syntaxin1a (Syx1a) and with an assembled SNARE complex containing Syx1a in an open conformation. The N-peptide of Syx1a (amino acids 1-24) has been implicated in the transition of Munc18a-bound Syx1a to Munc18a-bound SNARE complex, but the underlying mechanism is not understood. Here we report the X-ray crystal structures of Munc18a bound to Syx1a with and without its native N-peptide (Syx1aΔN), along with small-angle X-ray scattering (SAXS) data for Munc18a bound to Syx1a, Syx1aΔN, and Syx1a L165A/E166A (LE), a mutation thought to render Syx1a in a constitutively open conformation. We show that all three complexes adopt the same global structure, in which Munc18a binds a closed conformation of Syx1a. We also identify a possible structural connection between the Syx1a N-peptide and SNARE domain that might be important for the transition of closed-to-open Syx1a in SNARE complex assembly. Although the role of the N-peptide in Munc18a-mediated SNARE complex assembly remains unclear, our results demonstrate that the N-peptide and LE mutation have no effect on the global conformation of the Munc18a-Syx1a complex.
Organizational Affiliation:
Department of Structural Biology, Stanford University School of Medicine, Stanford, CA 94305, USA.