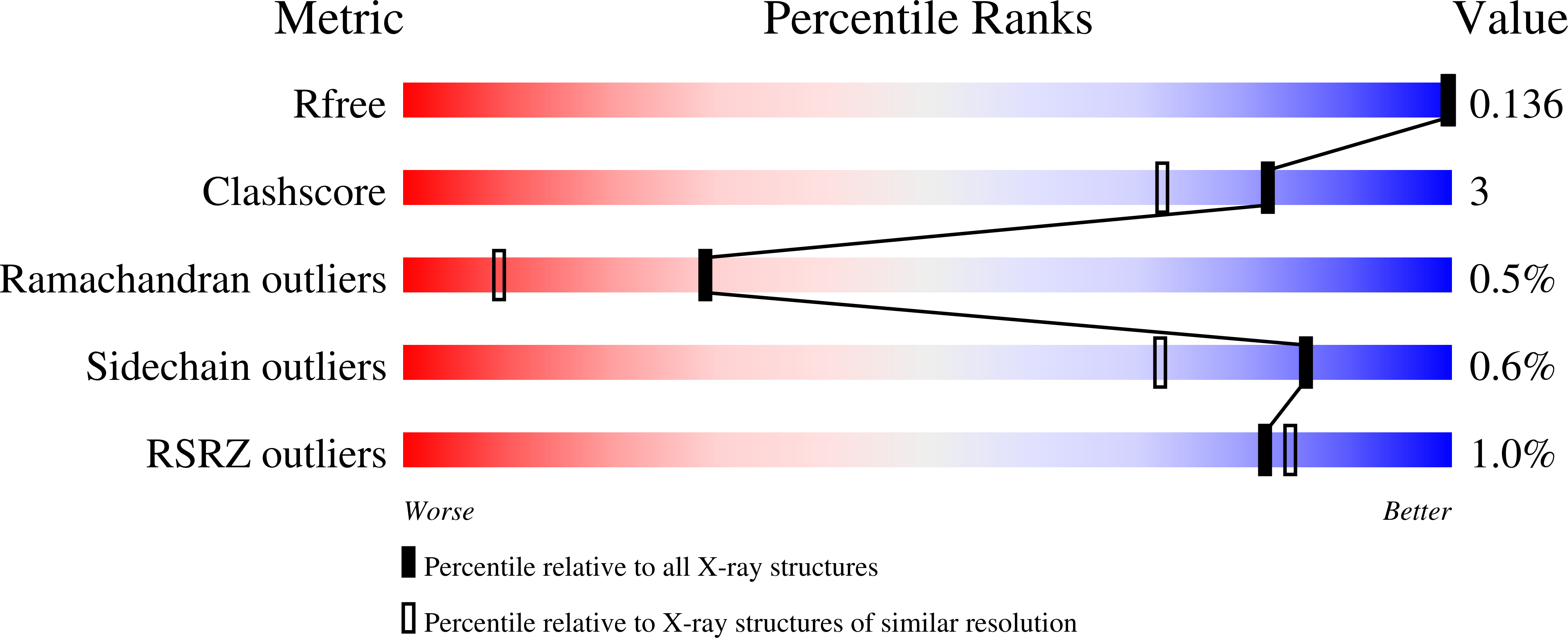
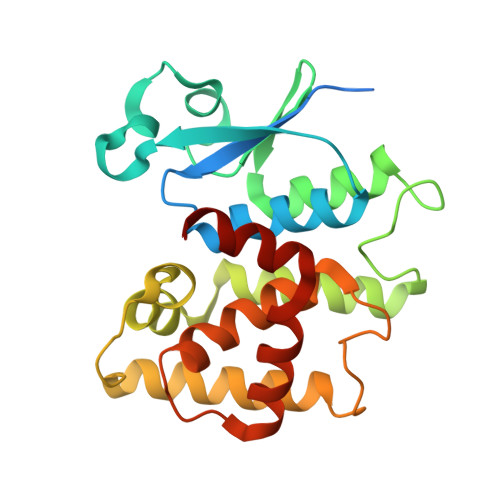
Crystal Structure of Glutathione S-Transferase A6Tby7 (Target Efi-507184) from Klebsiella Pneumoniae
Patskovsky, Y., Toro, R., Bhosle, R., Hillerich, B., Seidel, R.D., Washington, E., Scott Glenn, A., Chowdhury, S., Evans, B., Hammonds, J., Zencheck, W.D., Imker, H.J., Al Obaidi, N., Stead, M., Love, J., Gerlt, J.A., Armstrong, R.N., Almo, S.C., Enzyme Function Initiative (EFI)To be published.