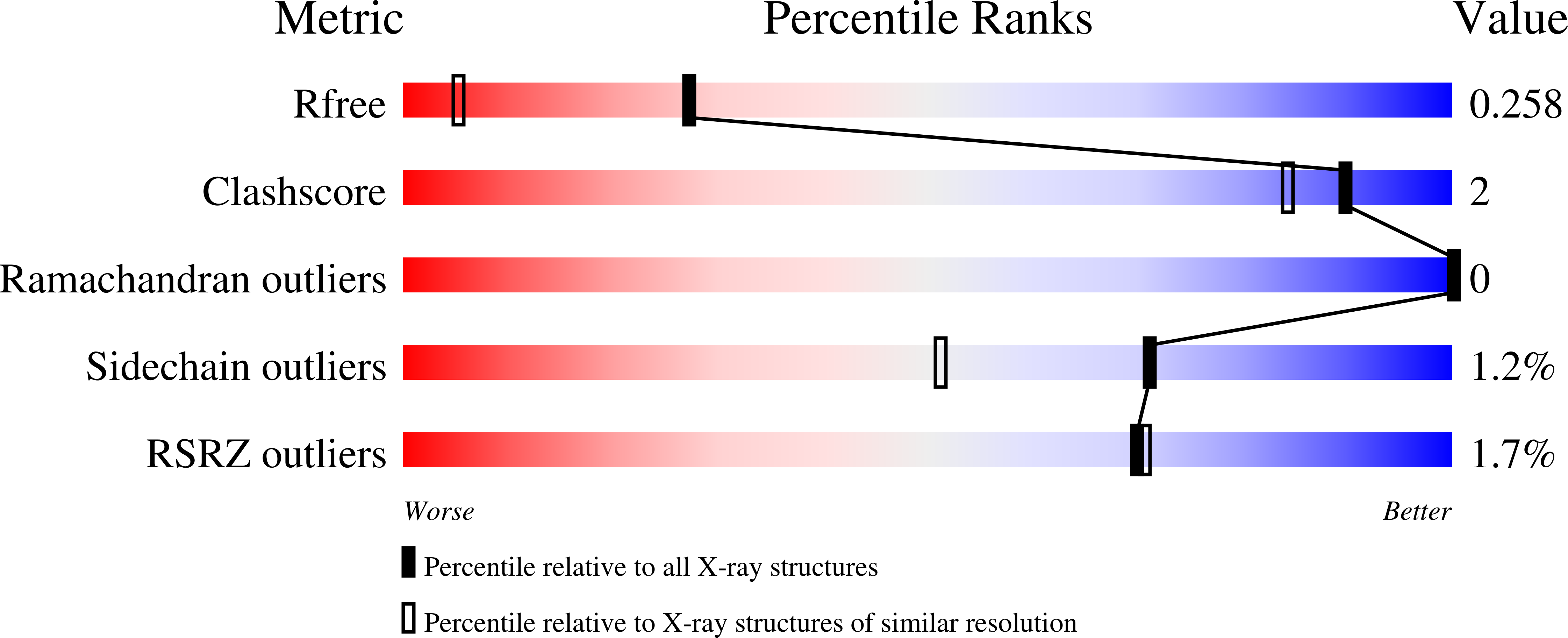
Structural basis for the oligomerization-state switch from a dimer to a trimer of an engineered cortexillin-1 coiled-coil variant.
Bjelic, S., Wieser, M., Frey, D., Stirnimann, C.U., Chance, M.R., Jaussi, R., Steinmetz, M.O., Kammerer, R.A.(2013) PLoS One 8: e63370-e63370
- PubMed: 23691037
- DOI: https://doi.org/10.1371/journal.pone.0063370
- Primary Citation of Related Structures:
4J4A - PubMed Abstract:
Coiled coils are well suited to drive subunit oligomerization and are widely used in applications ranging from basic research to medicine. The optimization of these applications requires a detailed understanding of the molecular determinants that control of coiled-coil formation. Although many of these determinants have been identified and characterized in great detail, a puzzling observation is that their presence does not necessarily correlate with the oligomerization state of a given coiled-coil structure. Thus, other determinants must play a key role. To address this issue, we recently investigated the unrelated coiled-coil domains from GCN4, ATF1 and cortexillin-1 as model systems. We found that well-known trimer-specific oligomerization-state determinants, such as the distribution of isoleucine residues at heptad-repeat core positions or the trimerization motif Arg-h-x-x-h-Glu (where h = hydrophobic amino acid; x = any amino acid), switch the peptide's topology from a dimer to a trimer only when inserted into the trigger sequence, a site indispensable for coiled-coil formation. Because high-resolution structural information could not be obtained for the full-length, three-stranded cortexillin-1 coiled coil, we here report the detailed biophysical and structural characterization of a shorter variant spanning the trigger sequence using circular dichroism, anatytical ultracentrifugation and x-ray crystallography. We show that the peptide forms a stable α-helical trimer in solution. We further determined the crystal structure of an optimised variant at a resolution of 1.65 Å, revealing that the peptide folds into a parallel, three-stranded coiled coil. The two complemented R-IxxIE trimerization motifs and the additional hydrophobic core isoleucine residue adopt the conformations seen in other extensively characterized parallel, three-stranded coiled coils. These findings not only confirm the structural basis for the switch in oligomerization state from a dimer to a trimer observed for the full-length cortexillin-1 coiled-coil domain, but also provide further evidence for a general link between oligomerization-state specificity and trigger-sequence function.
Organizational Affiliation:
Laboratory of Biomolecular Research, Paul Scherrer Institut, Villigen, Switzerland. sasa.bjelic@case.edu