Consistent mutational paths predict eukaryotic thermostability.
van Noort, V., Bradatsch, B., Arumugam, M., Amlacher, S., Bange, G., Creevey, C., Falk, S., Mende, D.R., Sinning, I., Hurt, E., Bork, P.(2013) BMC Evol Biol 13: 7-7
- PubMed: 23305080
- DOI: https://doi.org/10.1186/1471-2148-13-7
- Primary Citation of Related Structures:
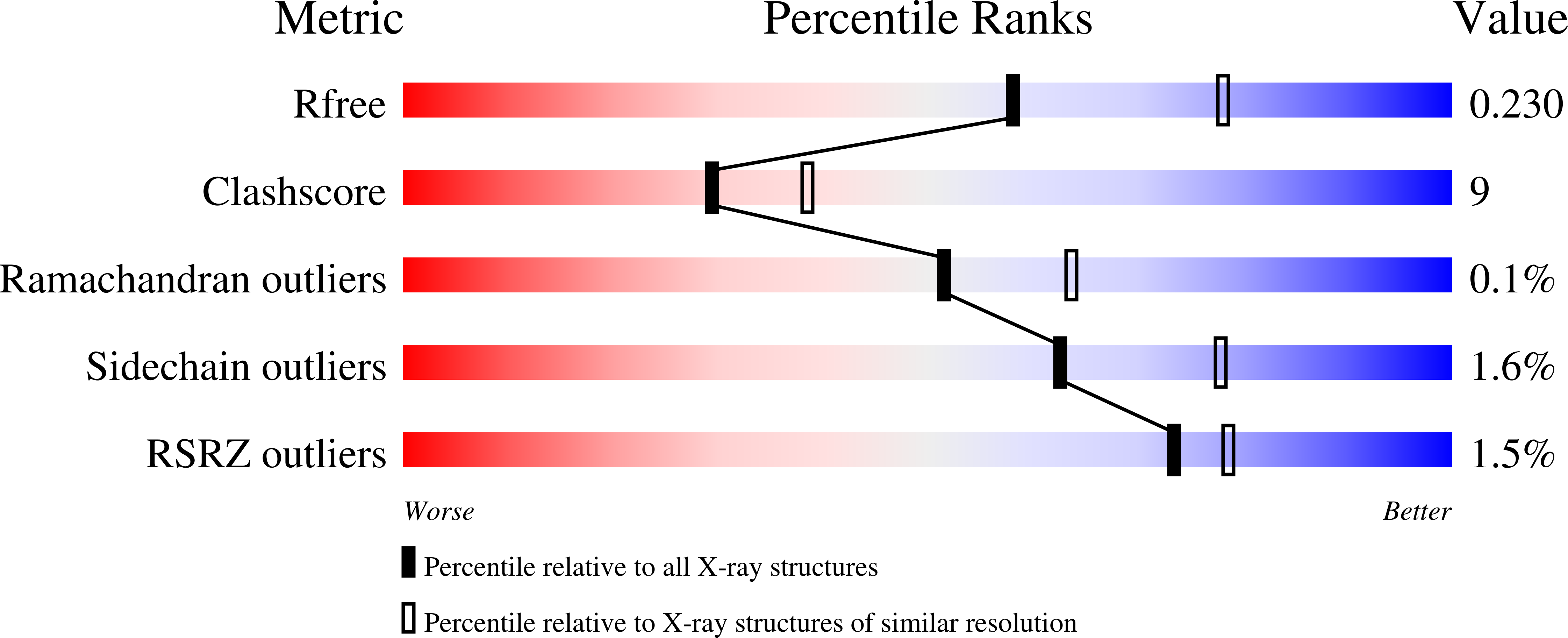
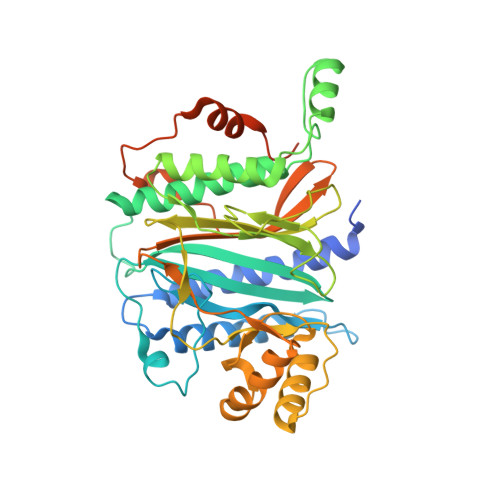
4IPA - PubMed Abstract:
Proteomes of thermophilic prokaryotes have been instrumental in structural biology and successfully exploited in biotechnology, however many proteins required for eukaryotic cell function are absent from bacteria or archaea. With Chaetomium thermophilum, Thielavia terrestris and Thielavia heterothallica three genome sequences of thermophilic eukaryotes have been published.
Organizational Affiliation:
European Molecular Biology Laboratory, Meyerhofstrasse 1, Heidelberg 69117, Germany.