The Structure of the Herpes Simplex Virus DNA-Packaging Terminase pUL15 Nuclease Domain Suggests an Evolutionary Lineage among Eukaryotic and Prokaryotic Viruses.
Selvarajan Sigamani, S., Zhao, H., Kamau, Y.N., Baines, J.D., Tang, L.(2013) J Virol 87: 7140-7148
- PubMed: 23596306
- DOI: https://doi.org/10.1128/JVI.00311-13
- Primary Citation of Related Structures:
4IOX - PubMed Abstract:
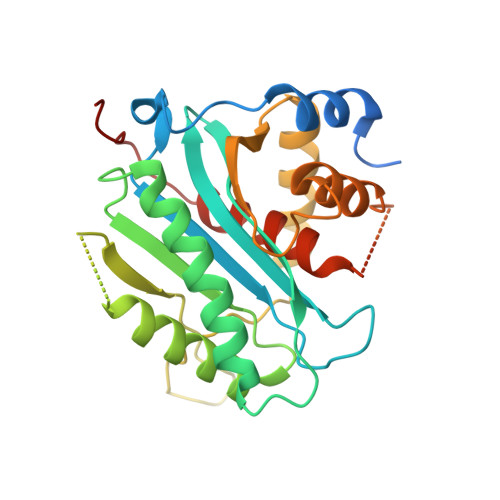
Herpes simplex virus 1 (HSV-1), the prototypic member of herpesviruses, employs a virally encoded molecular machine called terminase to package the viral double-stranded DNA (dsDNA) genome into a preformed protein shell. The terminase contains a large subunit that is thought to cleave concatemeric viral DNA during the packaging initiation and completion of each packaging cycle and supply energy to the packaging process via ATP hydrolysis. We have determined the X-ray structure of the C-terminal domain of the terminase large-subunit pUL15 (pUL15C) from HSV-1. The structure shows a fold resembling those of bacteriophage terminases, RNase H, integrases, DNA polymerases, and topoisomerases, with an active site clustered with acidic residues. Docking analysis reveals a DNA-binding surface surrounded by flexible loops, indicating considerable conformational changes upon DNA binding. In vitro assay shows that pUL15C possesses non-sequence-specific, Mg(2+)-dependent nuclease activity. These results suggest that pUL15 uses an RNase H-like, metal ion-mediated catalysis mechanism for cleavage of viral concatemeric DNA. The structure reveals extra structural elements in addition to the RNase H-like fold core and variations in local architecture of the nuclease active site, which are conserved in herpesvirus terminases and bear great similarity to the phage T4 gp17 but are distinct from podovirus and siphovirus orthologs and cellular RNase H, delineating a new evolutionary lineage among a large family of eukaryotic viruses and simple and complex prokaryotic viruses.
Organizational Affiliation:
Department of Molecular Biosciences, University of Kansas, Lawrence, Kansas, USA.