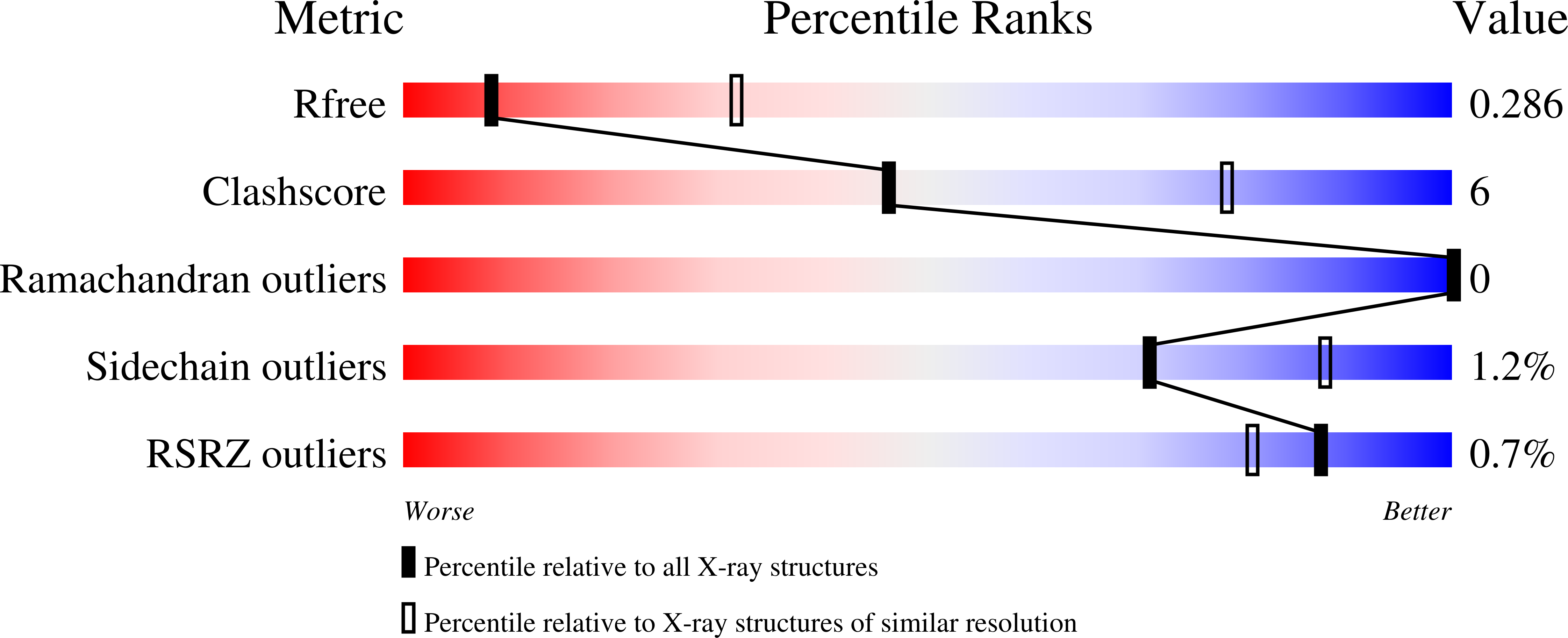
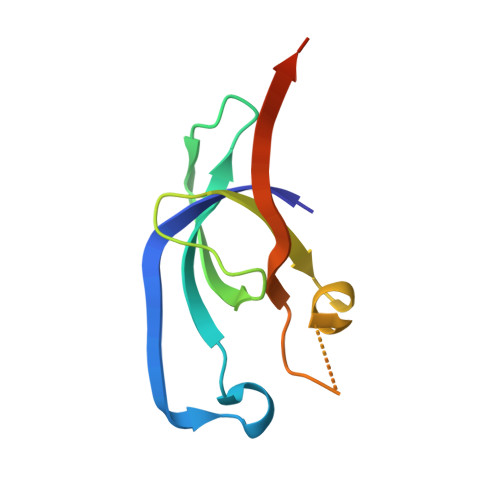
Bacterial microcompartment shells of diverse functional types possess pentameric vertex proteins.
Wheatley, N.M., Gidaniyan, S.D., Liu, Y., Cascio, D., Yeates, T.O.(2013) Protein Sci 22: 660-665
- PubMed: 23456886
- DOI: https://doi.org/10.1002/pro.2246
- Primary Citation of Related Structures:
4I7A - PubMed Abstract:
Bacterial microcompartments (MCPs) are large proteinaceous structures comprised of a roughly icosahedral shell and a series of encapsulated enzymes. MCPs carrying out three different metabolic functions have been characterized in some detail, while gene expression and bioinformatics studies have implicated other types, including one believed to perform glycyl radical-based metabolism of 1,2-propanediol (Grp). Here we report the crystal structure of a protein (GrpN), which is presumed to be part of the shell of a Grp-type MCP in Rhodospirillum rubrum F11. GrpN is homologous to a family of proteins (EutN/PduN/CcmL/CsoS4) whose members have been implicated in forming the vertices of MCP shells. Consistent with that notion, the crystal structure of GrpN revealed a pentameric assembly. That observation revived an outstanding question about the oligomeric state of this protein family: pentameric forms (for CcmL and CsoS4A) and a hexameric form (for EutN) had both been observed in previous crystal structures. To clarify these confounding observations, we revisited the case of EutN. We developed a molecular biology-based method for accurately determining the number of subunits in homo-oligomeric proteins, and found unequivocally that EutN is a pentamer in solution. Based on these convergent findings, we propose the name bacterial microcompartment vertex for this special family of MCP shell proteins.
Organizational Affiliation:
Molecular Biology Institute, University of California, Los Angeles, California, USA.