Computational, structural, and kinetic evidence that Vibrio vulnificus FrsA is not a cofactor-independent pyruvate decarboxylase.
Kellett, W.F., Brunk, E., Desai, B.J., Fedorov, A.A., Almo, S.C., Gerlt, J.A., Rothlisberger, U., Richards, N.G.(2013) Biochemistry 52: 1842-1844
- PubMed: 23452154
- DOI: https://doi.org/10.1021/bi400093y
- Primary Citation of Related Structures:
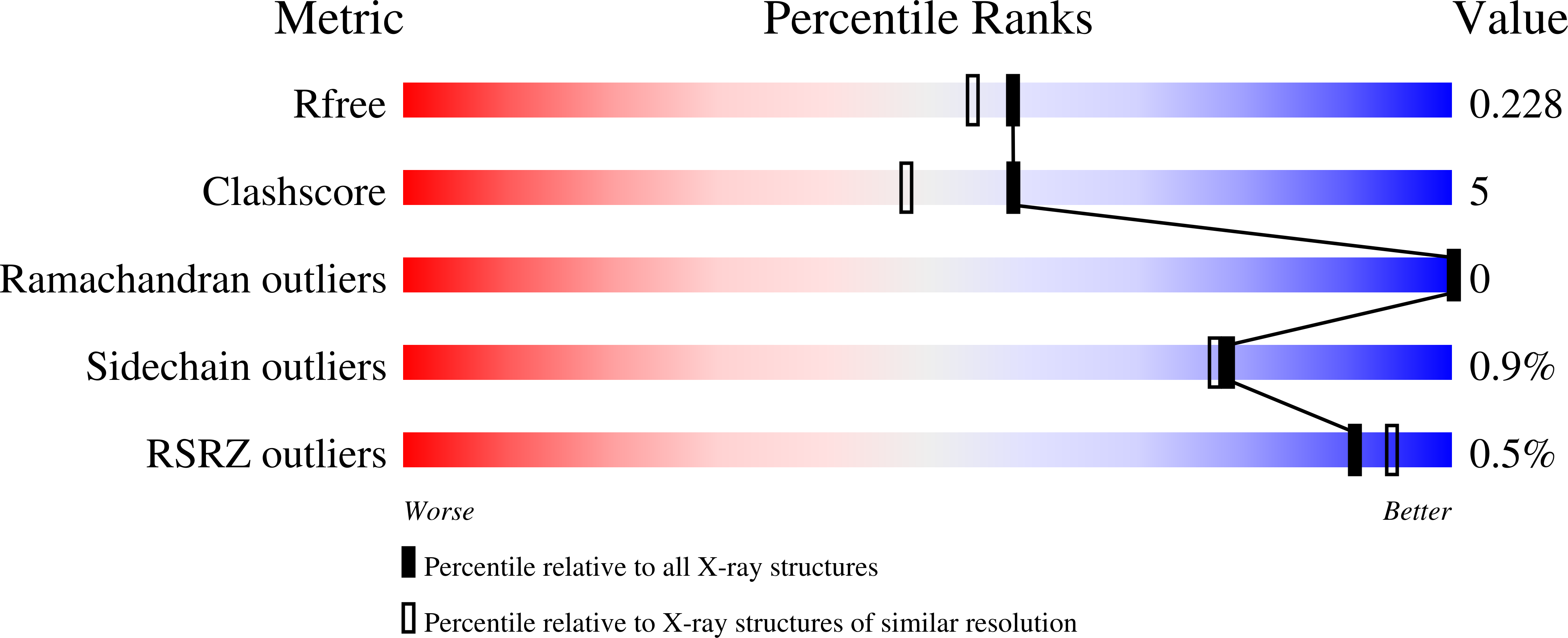
4I4C - PubMed Abstract:
The fermentation-respiration switch (FrsA) protein in Vibrio vulnificus was recently reported to catalyze the cofactor-independent decarboxylation of pyruvate. We now report quantum mechanical/molecular mechenical calculations that examine the energetics of C-C bond cleavage for a pyruvate molecule bound within the putative active site of FrsA. These calculations suggest that the barrier to C-C bond cleavage in the bound substrate is 28 kcal/mol, which is similar to that estimated for the uncatalyzed decarboxylation of pyruvate in water at 25 °C. In agreement with the theoretical predictions, no pyruvate decarboxylase activity was detected for recombinant FrsA protein that could be crystallized and structurally characterized. These results suggest that the functional annotation of FrsA as a cofactor-independent pyruvate decarboxylase is incorrect.
Organizational Affiliation:
Department of Chemistry, University of Florida, Gainesville, Florida 32611, United States.