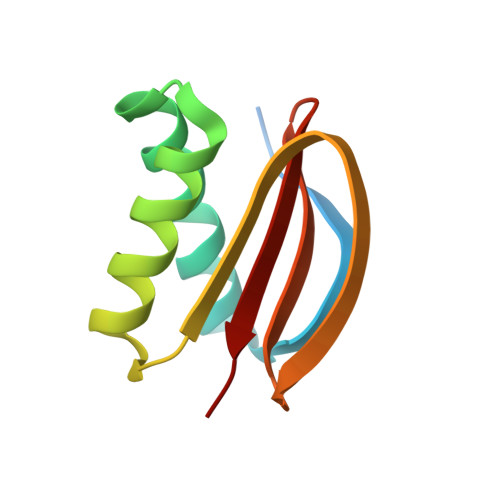
The yeast dynein Dyn2-Pac11 complex is a dynein dimerization/processivity factor: structural and single-molecule characterization.
Rao, L., Romes, E.M., Nicholas, M.P., Brenner, S., Tripathy, A., Gennerich, A., Slep, K.C.(2013) Mol Biol Cell 24: 2362-2377
- PubMed: 23761070
- DOI: https://doi.org/10.1091/mbc.E13-03-0166
- Primary Citation of Related Structures:
4HT6 - PubMed Abstract:
Cytoplasmic dynein is the major microtubule minus end-directed motor. Although studies have probed the mechanism of the C-terminal motor domain, if and how dynein's N-terminal tail and the accessory chains it binds regulate motor activity remain to be determined. Here, we investigate the structure and function of the Saccharomyces cerevisiae dynein light (Dyn2) and intermediate (Pac11) chains in dynein heavy chain (Dyn1) movement. We present the crystal structure of a Dyn2-Pac11 complex, showing Dyn2-mediated Pac11 dimerization. To determine the molecular effects of Dyn2 and Pac11 on Dyn1 function, we generated dyn2Δ and dyn2Δpac11Δ strains and analyzed Dyn1 single-molecule motor activity. We find that the Dyn2-Pac11 complex promotes Dyn1 homodimerization and potentiates processivity. The absence of Dyn2 and Pac11 yields motors with decreased velocity, dramatically reduced processivity, increased monomerization, aggregation, and immobility as determined by single-molecule measurements. Deleting dyn2 significantly reduces Pac11-Dyn1 complex formation, yielding Dyn1 motors with activity similar to Dyn1 from the dyn2Δpac11Δ strain. Of interest, motor phenotypes resulting from Dyn2-Pac11 complex depletion bear similarity to a point mutation in the mammalian dynein N-terminal tail (Loa), highlighting this region as a conserved, regulatory motor element.
Organizational Affiliation:
Department of Anatomy and Structural Biology and Gruss Lipper Biophotonics Center, Albert Einstein College of Medicine, New York, NY 10461, USA.