2.2A resolution structure of Proteasome Assembly Chaperone Hsm3
Singh, R., Lovell, S., Zolkiewski, M., Battaile, K.P., Roelofs, J.To be published.
Experimental Data Snapshot
wwPDB Validation 3D Report Full Report
Entity ID: 1 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| DNA mismatch repair protein HSM3 | 491 | Saccharomyces cerevisiae S288C | Mutation(s): 0 Gene Names: HSM3, YBR272C, YBR1740 | 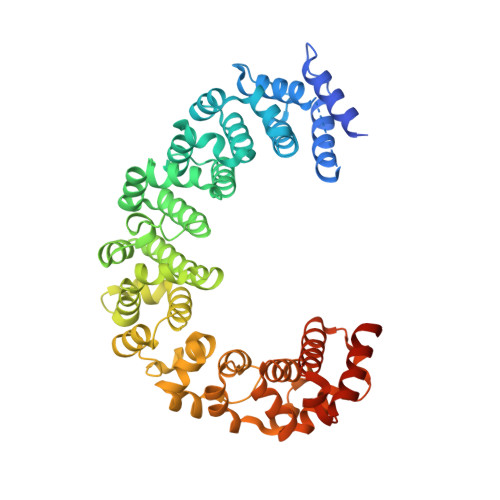 | |
UniProt | |||||
Find proteins for P38348 (Saccharomyces cerevisiae (strain ATCC 204508 / S288c)) Explore P38348 Go to UniProtKB: P38348 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | P38348 | ||||
Sequence AnnotationsExpand | |||||
| |||||
| Length ( Å ) | Angle ( ˚ ) |
|---|---|
| a = 85.148 | α = 90 |
| b = 94.466 | β = 90 |
| c = 129.931 | γ = 90 |
| Software Name | Purpose |
|---|---|
| SCALA | data scaling |
| PHASER | phasing |
| PHENIX | refinement |
| PDB_EXTRACT | data extraction |
| JDirector | data collection |
| XDS | data reduction |
| PHENIX | phasing |