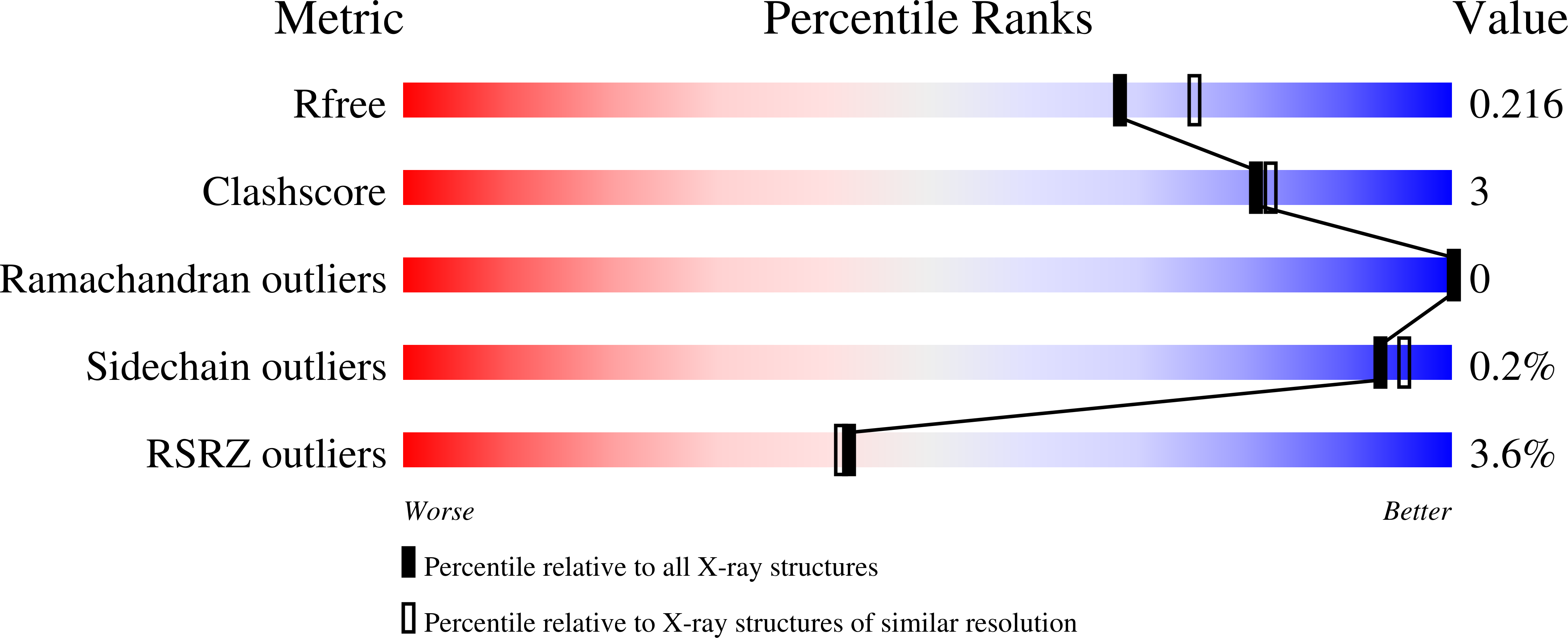
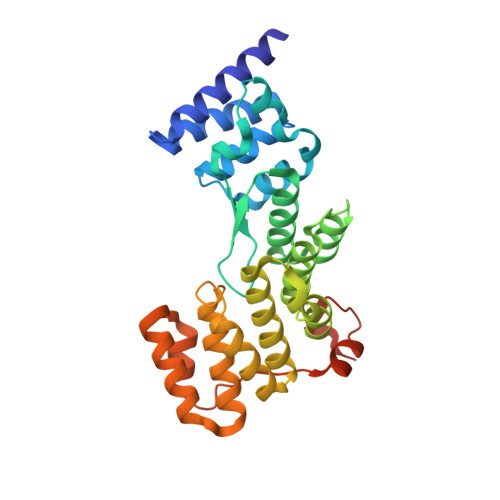
Crystal structure of the N-terminal domain of EccA1 ATPase from the ESX-1 secretion system of Mycobacterium tuberculosis.
Wagner, J.M., Evans, T.J., Korotkov, K.V.(2014) Proteins 82: 159-163
- PubMed: 23818233
- DOI: https://doi.org/10.1002/prot.24351
- Primary Citation of Related Structures:
4F3V - PubMed Abstract:
EccA1 is an important component of the type VII secretion system (T7SS) that is responsible for transport of virulence factors in pathogenic mycobacteria. EccA1 has an N-terminal domain of unknown function and a C-terminal AAA+ (ATPases associated with various cellular activities) domain. Here we report the crystal structure of the N-terminal domain of EccA1 from Mycobacterium tuberculosis, which shows an arrangement of six tetratricopeptide repeats that may mediate interactions of EccA1 with secreted substrates. Furthermore, the size and shape of the N-terminal domain suggest its orientation in the context of a hexamer model of full-length EccA1.
Organizational Affiliation:
Department of Molecular and Cellular Biochemistry and Center for Structural Biology, University of Kentucky, Lexington, Kentucky, 40536.