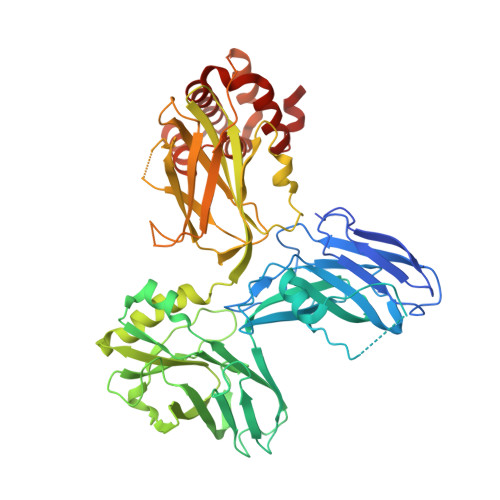
Structure of the ZU5-ZU5-UPA-DD tandem of ankyrin-B reveals interaction surfaces necessary for ankyrin function
Wang, C., Yu, C., Ye, F., Wei, Z., Zhang, M.(2012) Proc Natl Acad Sci U S A 109: 4822-4827
- PubMed: 22411828
- DOI: https://doi.org/10.1073/pnas.1200613109
- Primary Citation of Related Structures:
4D8O - PubMed Abstract:
Ankyrin-R/B/G (encoded by ANK1/2/3, respectively) are a family of very large scaffold proteins capable of anchoring numerous receptors and ion channels to specific, spectrin-containing membrane micro-domains. Hereditary mutations of ankyrins are known to be associated with diseases including spherocytosis, cardiac arrhythmia, and bipolar disorder in humans, although the underlying molecular bases are poorly understood. The middle spectrin-binding domain of ankyrins contains highly conserved ZU5-ZU5-UPA-DD domains arranged into the ZZUD tandem. Curiously, most of the disease-causing mutations in the tandem have no apparent impact on the spectrin binding of ankyrins. The high resolution structure of the ankyrin-B ZZUD tandem determined here reveals that the ZU5-ZU5-UPA domains form a tightly packed structural supramodule, whereas DD is freely accessible. Although the formation of the ZZU supramodule does not influence the spectrin binding of ankyrins, mutations altering the interdomain interfaces of ZZU impair the functions of ankyrin-B&G. Our structural analysis further indicates that the ZZU supramodule of ankyrins has two additional surfaces that may bind to targets other than spectrin. Finally, the structure of the ankyrin ZZUD provides mechanistic explanations to many disease-causing mutations identified in ankyrin-B&R.
Organizational Affiliation:
Division of Life Science, State Key Laboratory of Molecular Neuroscience, Hong Kong University of Science and Technology, Clear Water Bay, Kowloon, Hong Kong.