A Detailed Look Into Chinese Hamster Legumain Active Site Structure and Exploration of its Function
Li, W., Damme, M., Buessow, K., Grimm, I., Van Den Heuvel, J., Heinz, D.W., Krausze, J.To be published.
Experimental Data Snapshot
Entity ID: 1 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| PROLEGUMAIN | 408 | Cricetulus griseus | Mutation(s): 0 EC: 3.4.22.34 | 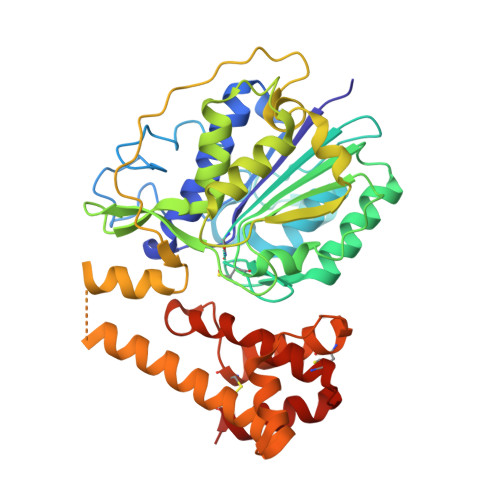 | |
UniProt | |||||
Find proteins for G3I1H5 (Cricetulus griseus) Explore G3I1H5 Go to UniProtKB: G3I1H5 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | G3I1H5 | ||||
Sequence AnnotationsExpand | |||||
| |||||
| Ligands 1 Unique | |||||
|---|---|---|---|---|---|
| ID | Chains | Name / Formula / InChI Key | 2D Diagram | 3D Interactions | |
| NAG Query on NAG | B [auth A], C [auth A], D [auth A] | 2-acetamido-2-deoxy-beta-D-glucopyranose C8 H15 N O6 OVRNDRQMDRJTHS-FMDGEEDCSA-N |  | ||
| Modified Residues 2 Unique | |||||
|---|---|---|---|---|---|
| ID | Chains | Type | Formula | 2D Diagram | Parent |
| CSX Query on CSX | A | L-PEPTIDE LINKING | C3 H7 N O3 S |  | CYS |
| SNN Query on SNN | A | L-PEPTIDE LINKING | C4 H6 N2 O2 |  | ASN |
| Length ( Å ) | Angle ( ˚ ) |
|---|---|
| a = 50.81 | α = 90 |
| b = 50.81 | β = 90 |
| c = 253.59 | γ = 120 |
| Software Name | Purpose |
|---|---|
| PHENIX | refinement |
| XDS | data reduction |
| XDS | data scaling |
| PHASER | phasing |