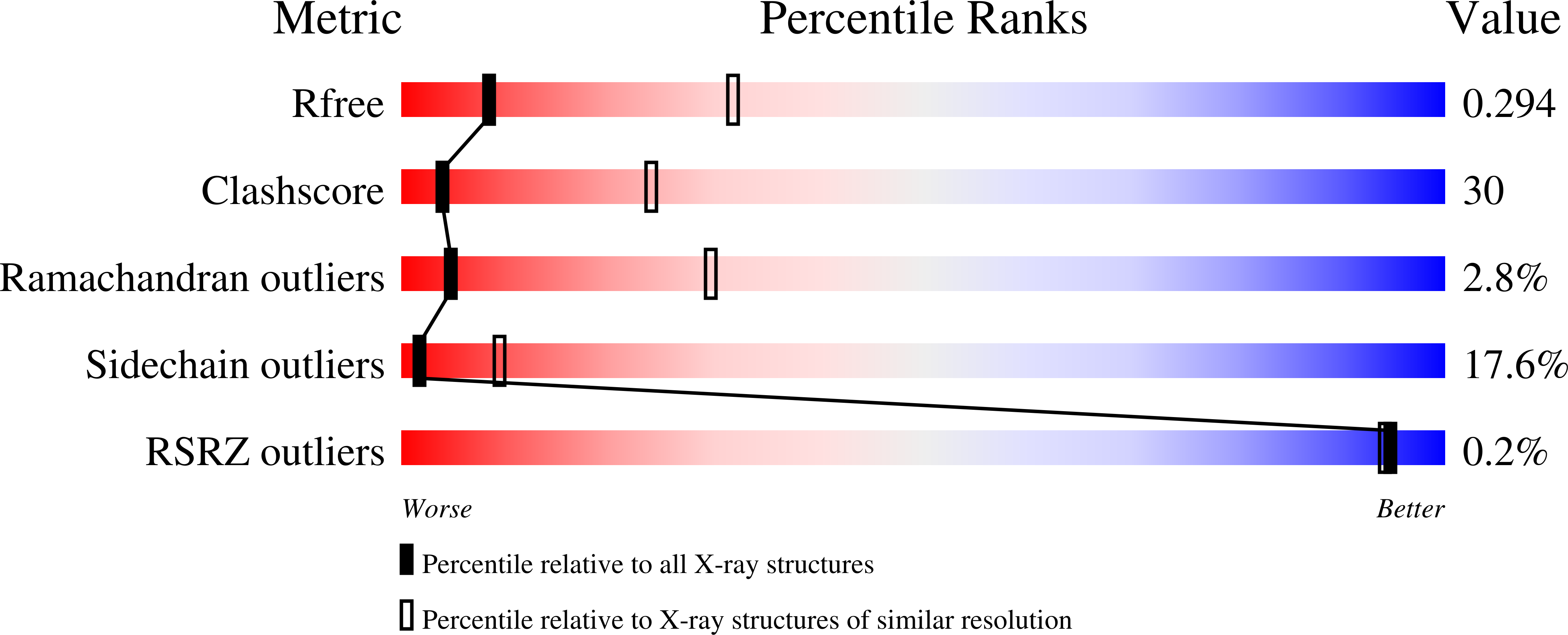
Crenactin from Pyrobaculum Calidifontis is Closely Related to Actin in Structure and Forms Steep Helical Filaments.
Izore, T., Duman, R., Kureisaite-Ciziene, D., Lowe, J.(2014) FEBS Lett 588: 776
- PubMed: 24486010
- DOI: https://doi.org/10.1016/j.febslet.2014.01.029
- Primary Citation of Related Structures:
4CJ7 - PubMed Abstract:
Polymerising proteins of the actin family are nearly ubiquitous. Crenactins, restricted to Crenarchaea, are more closely related to actin than bacterial MreB. Crenactins occur in gene clusters hinting at an unknown, but conserved function. We solved the crystal structure of crenactin at 3.2 Å resolution. The protein crystallises as a continuous right-handed helix with 8 subunits per complete turn, spanning 419 Å. The structure of crenactin shows several loops that are longer than in actin, but overall, crenactin is closely related to eukaryotic actin, with an RMSD of 1.6 Å. Crenactin filaments imaged by electron microscopy showed polymers with very similar helical parameters.
Organizational Affiliation:
MRC Laboratory of Molecular Biology, Structural Studies Division, Francis Crick Avenue, Cambridge CB2 0QH, United Kingdom.