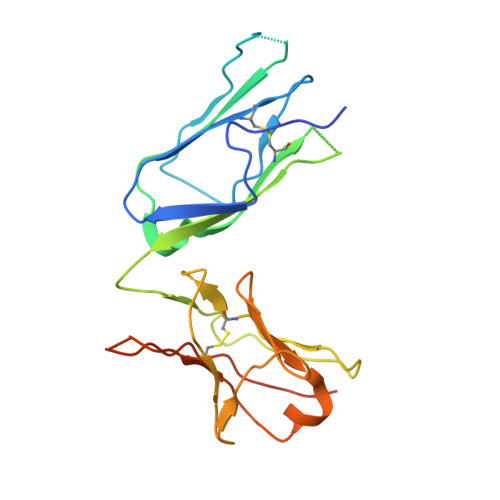
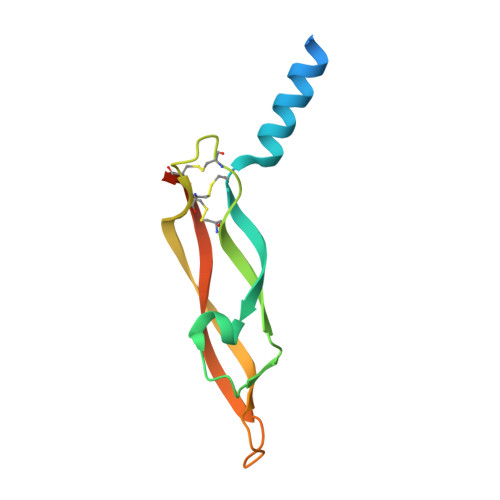
Structural and Mechanistic Insights Into Vegfr-3 Ligand Binding and Activation
Leppanen, V.-M., Tvorogov, D., Kisko, K., Prota, A.E., Jeltsch, M., Anisimov, A., Markovic-Mueller, S., Stuttfeld, E., Goldie, K.N., Ballmer-Hofer, K., Alitalo, K.(2013) Proc Natl Acad Sci U S A 110: 12960
- PubMed: 23878260
- DOI: https://doi.org/10.1073/pnas.1301415110
- Primary Citation of Related Structures:
4BSJ, 4BSK - PubMed Abstract:
Vascular endothelial growth factors (VEGFs) and their receptors (VEGFRs) are key drivers of blood and lymph vessel formation in development, but also in several pathological processes. VEGF-C signaling through VEGFR-3 promotes lymphangiogenesis, which is a clinically relevant target for treating lymphatic insufficiency and for blocking tumor angiogenesis and metastasis. The extracellular domain of VEGFRs consists of seven Ig homology domains; domains 1-3 (D1-3) are responsible for ligand binding, and the membrane-proximal domains 4-7 (D4-7) are involved in structural rearrangements essential for receptor dimerization and activation. Here we analyzed the crystal structures of VEGF-C in complex with VEGFR-3 domains D1-2 and of the VEGFR-3 D4-5 homodimer. The structures revealed a conserved ligand-binding interface in D2 and a unique mechanism for VEGFR dimerization and activation, with homotypic interactions in D5. Mutation of the conserved residues mediating the D5 interaction (Thr446 and Lys516) and the D7 interaction (Arg737) compromised VEGF-C induced VEGFR-3 activation. A thermodynamic analysis of VEGFR-3 deletion mutants showed that D3, D4-5, and D6-7 all contribute to ligand binding. A structural model of the VEGF-C/VEGFR-3 D1-7 complex derived from small-angle X-ray scattering data is consistent with the homotypic interactions in D5 and D7. Taken together, our data show that ligand-dependent homotypic interactions in D5 and D7 are essential for VEGFR activation, opening promising possibilities for the design of VEGFR-specific drugs.
Organizational Affiliation:
Translational Cancer Biology Program, Institute for Molecular Medicine Finland and Helsinki University Central Hospital, Biomedicum Helsinki, University of Helsinki, 00014 Helsinki, Finland.