Direct Interaction of Actin Filaments with F-Bar Protein Pacsin2.
Kostan, J., Salzer, U., Orlova, A., Toro, I., Hodnik, V., Senju, Y., Zou, J., Schreiner, C., Steiner, J., Merilainen, J., Nikki, M., Virtanen, I., Carugo, O., Rappsilber, J., Lappalainen, P., Lehto, V., Anderluh, G., Egelman, E.H., Djinovic-Carugo, K.(2014) EMBO Rep 15: 1154
- PubMed: 25216944
- DOI: https://doi.org/10.15252/embr.201439267
- Primary Citation of Related Structures:
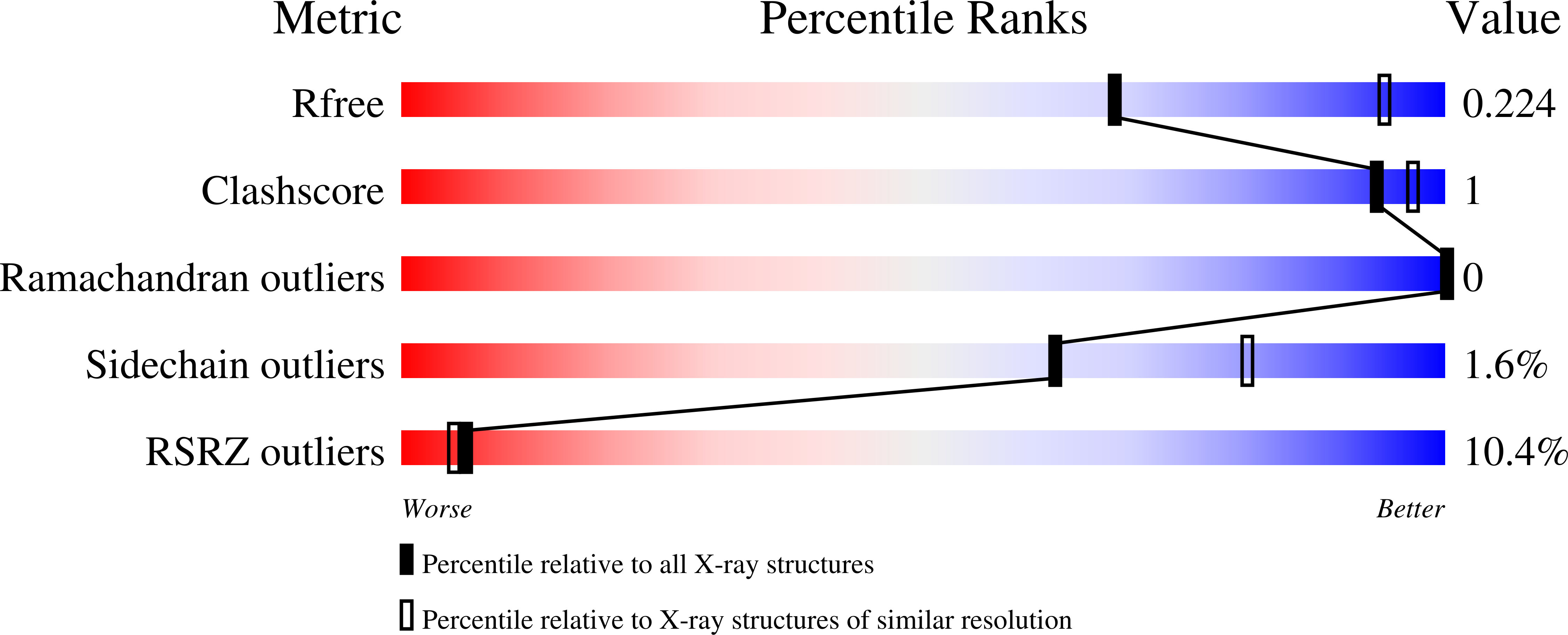
4BNE - PubMed Abstract:
Two mechanisms have emerged as major regulators of membrane shape: BAR domain-containing proteins, which induce invaginations and protrusions, and nuclear promoting factors, which cause generation of branched actin filaments that exert mechanical forces on membranes. While a large body of information exists on interactions of BAR proteins with membranes and regulatory proteins of the cytoskeleton, little is known about connections between these two processes. Here, we show that the F-BAR domain protein pacsin2 is able to associate with actin filaments using the same concave surface employed to bind to membranes, while some other tested N-BAR and F-BAR proteins (endophilin, CIP4 and FCHO2) do not associate with actin. This finding reveals a new level of complexity in membrane remodeling processes.
Organizational Affiliation:
Department of Structural and Computational Biology, Max F. Perutz Laboratories, University of Vienna, Vienna, Austria.