Nitrate in the Active Site of Protein Tyrosine Phosphatase 1B is a Putative Mimetic of the Transition State.
Kenny, P.W., Newman, J., Peat, T.S.(2014) Acta Crystallogr D Biol Crystallogr 70: 565
- PubMed: 24531490
- DOI: https://doi.org/10.1107/S1399004713031052
- Primary Citation of Related Structures:
4BJO - PubMed Abstract:
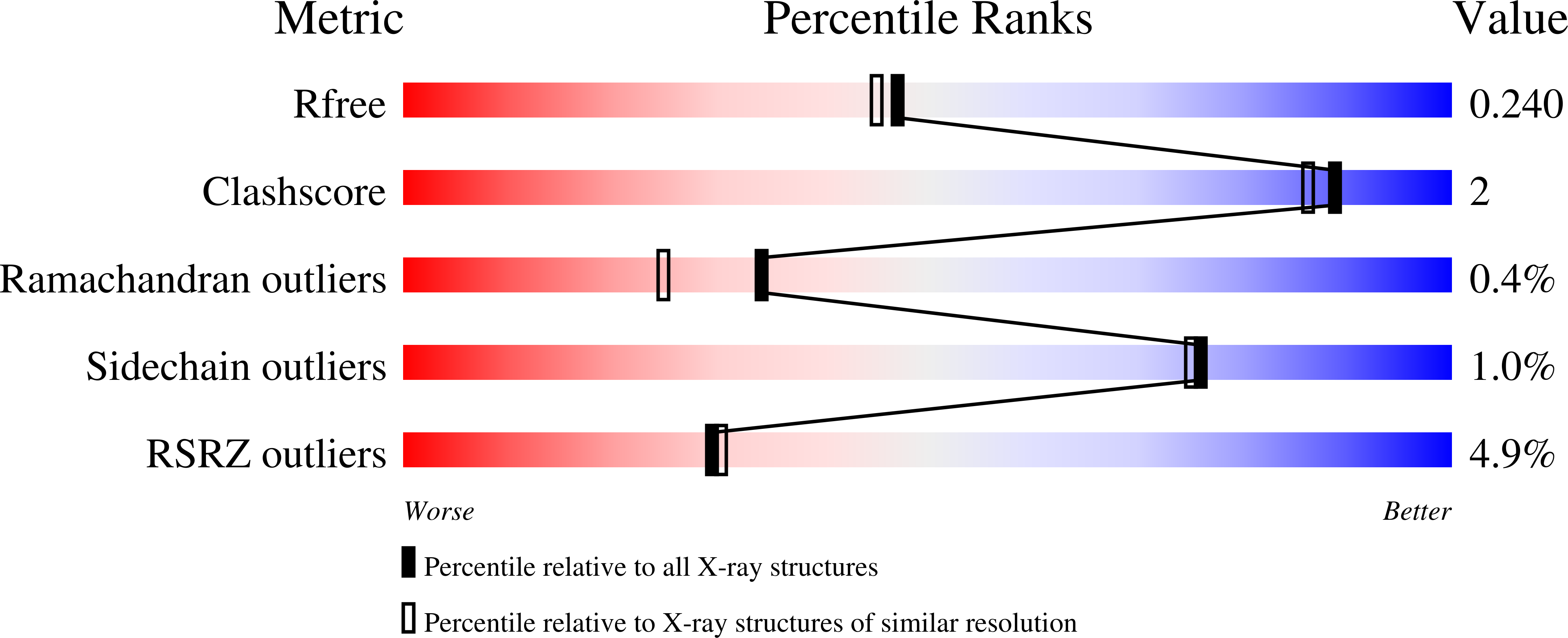
The X-ray crystal structure of the complex of protein tyrosine phosphatase 1B with nitrate anion has been determined and modelled quantum-mechanically. Two protomers were present in the structure, one with the mechanistically important WPD loop closed and the other with this loop open. Nitrate was observed bound to each protomer, making close contacts with the S atom of the catalytic cysteine and a tyrosine residue from a crystallographically related protomer.
Organizational Affiliation:
CSIRO Materials, Science and Engineering, 343 Royal Parade, Parkville, VIC 3052, Australia.