High-Resolution Structure and Mechanism of an F/V-Hybrid Rotor Ring in a Na+-Coupled ATP Synthase
Matthies, D., Zhou, W., Klyszejko, A.L., Anselmi, C., Yildiz, O., Brandt, K., Muller, V., Faraldo-Gomez, J.D., Meier, T.(2014) Nat Commun 5: 5286
- PubMed: 25381992
- DOI: https://doi.org/10.1038/ncomms6286
- Primary Citation of Related Structures:
4BEM - PubMed Abstract:
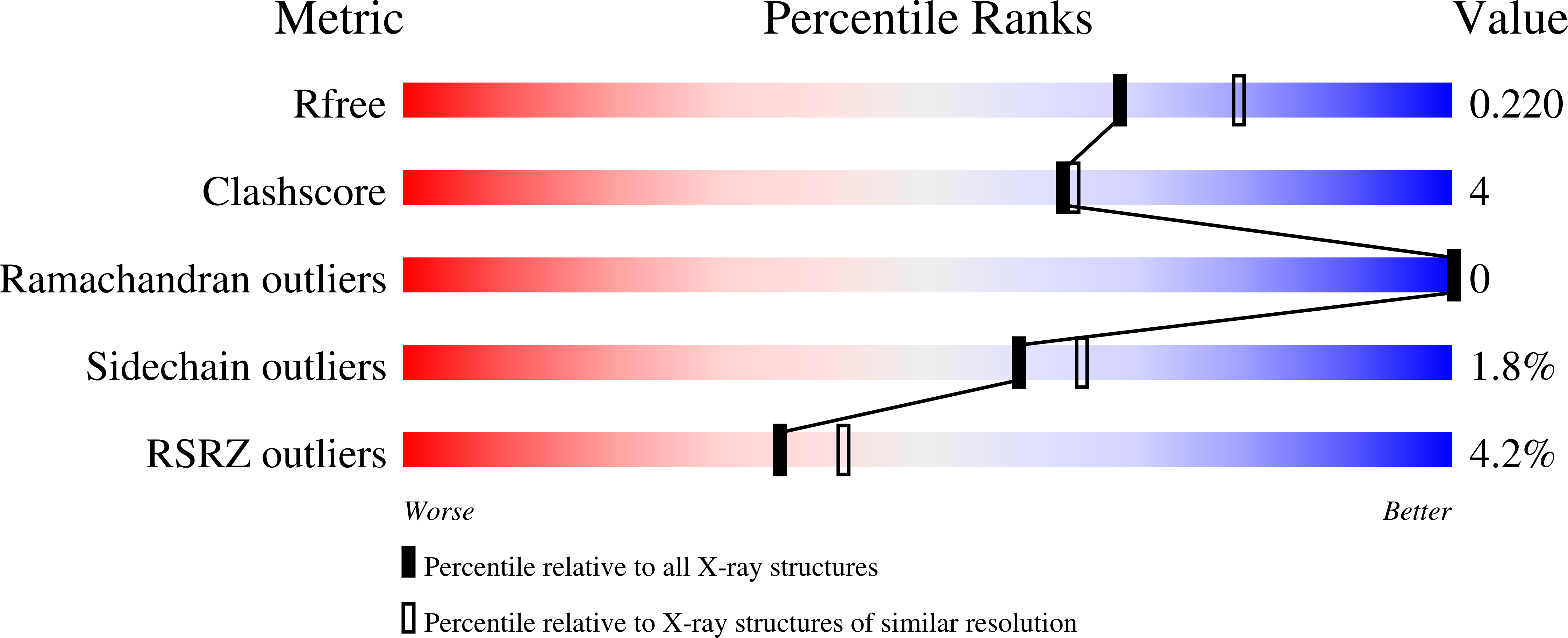
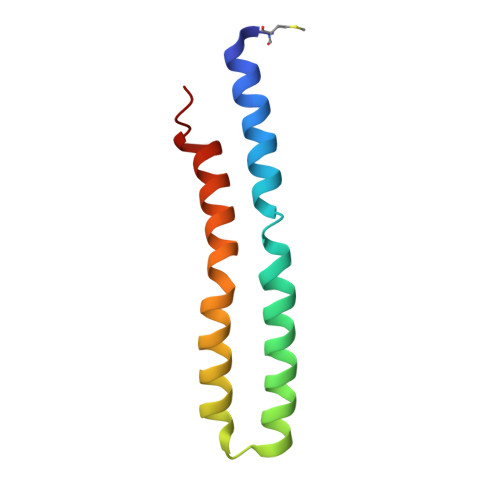
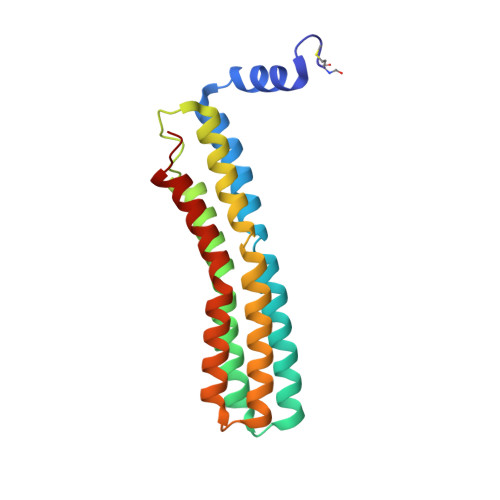
All rotary ATPases catalyse the interconversion of ATP and ADP-Pi through a mechanism that is coupled to the transmembrane flow of H(+) or Na(+). Physiologically, however, F/A-type enzymes specialize in ATP synthesis driven by downhill ion diffusion, while eukaryotic V-type ATPases function as ion pumps. To begin to rationalize the molecular basis for this functional differentiation, we solved the crystal structure of the Na(+)-driven membrane rotor of the Acetobacterium woodii ATP synthase, at 2.1 Å resolution. Unlike known structures, this rotor ring is a 9:1 heteromer of F- and V-type c-subunits and therefore features a hybrid configuration of ion-binding sites along its circumference. Molecular and kinetic simulations are used to dissect the mechanisms of Na(+) recognition and rotation of this c-ring, and to explain the functional implications of the V-type c-subunit. These structural and mechanistic insights indicate an evolutionary path between synthases and pumps involving adaptations in the rotor ring.
Organizational Affiliation:
Department of Structural Biology, Max Planck Institute of Biophysics, Max-von-Laue-Str. 3, 60438 Frankfurt am Main, Germany.