Structural and Biochemical Analysis of an Aurora B Kinase Mutant Reveals a Multistep Activation Mechanism
Sessa, F., Villa, F.To be published.
Experimental Data Snapshot
Entity ID: 1 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| AURORA KINASE B-A | 286 | Xenopus laevis | Mutation(s): 0 EC: 2.7.11.1 | 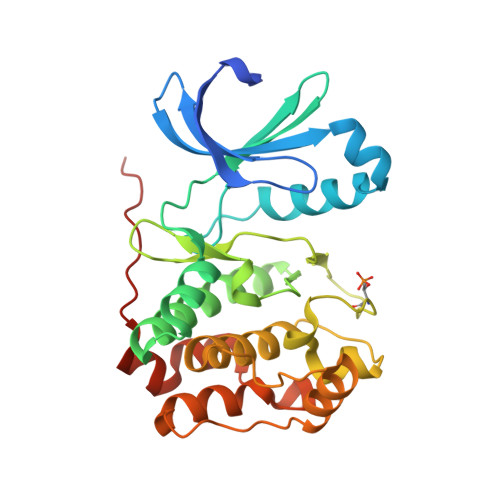 | |
UniProt | |||||
Find proteins for Q6DE08 (Xenopus laevis) Explore Q6DE08 Go to UniProtKB: Q6DE08 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | Q6DE08 | ||||
Sequence AnnotationsExpand | |||||
| |||||
Entity ID: 2 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| INNER CENTROMERE PROTEIN A | 44 | Xenopus laevis | Mutation(s): 0 | 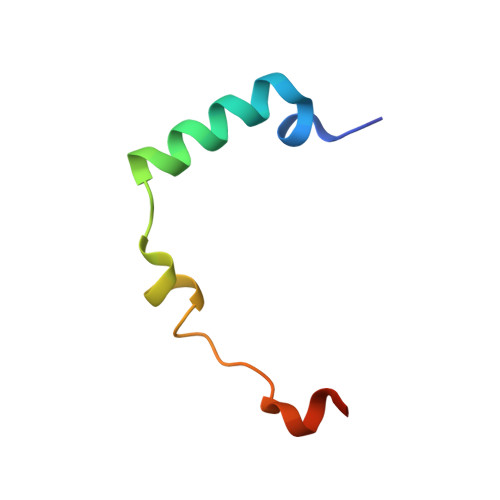 | |
UniProt | |||||
Find proteins for O13024 (Xenopus laevis) Explore O13024 Go to UniProtKB: O13024 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | O13024 | ||||
Sequence AnnotationsExpand | |||||
| |||||
| Ligands 1 Unique | |||||
|---|---|---|---|---|---|
| ID | Chains | Name / Formula / InChI Key | 2D Diagram | 3D Interactions | |
| VX6 Query on VX6 | E [auth A], F [auth B] | CYCLOPROPANECARBOXYLIC ACID {4-[4-(4-METHYL-PIPERAZIN-1-YL)-6-(5-METHYL-2H-PYRAZOL-3-YLAMINO)-PYRIMIDIN-2-YLSULFANYL]-PHENYL}-AMIDE C23 H28 N8 O S GCIKSSRWRFVXBI-UHFFFAOYSA-N |  | ||
| Modified Residues 1 Unique | |||||
|---|---|---|---|---|---|
| ID | Chains | Type | Formula | 2D Diagram | Parent |
| TPO Query on TPO | A, B | L-PEPTIDE LINKING | C4 H10 N O6 P |  | THR |
| Length ( Å ) | Angle ( ˚ ) |
|---|---|
| a = 45.787 | α = 90 |
| b = 67.259 | β = 96.58 |
| c = 116.755 | γ = 90 |
| Software Name | Purpose |
|---|---|
| REFMAC | refinement |
| MOLREP | phasing |