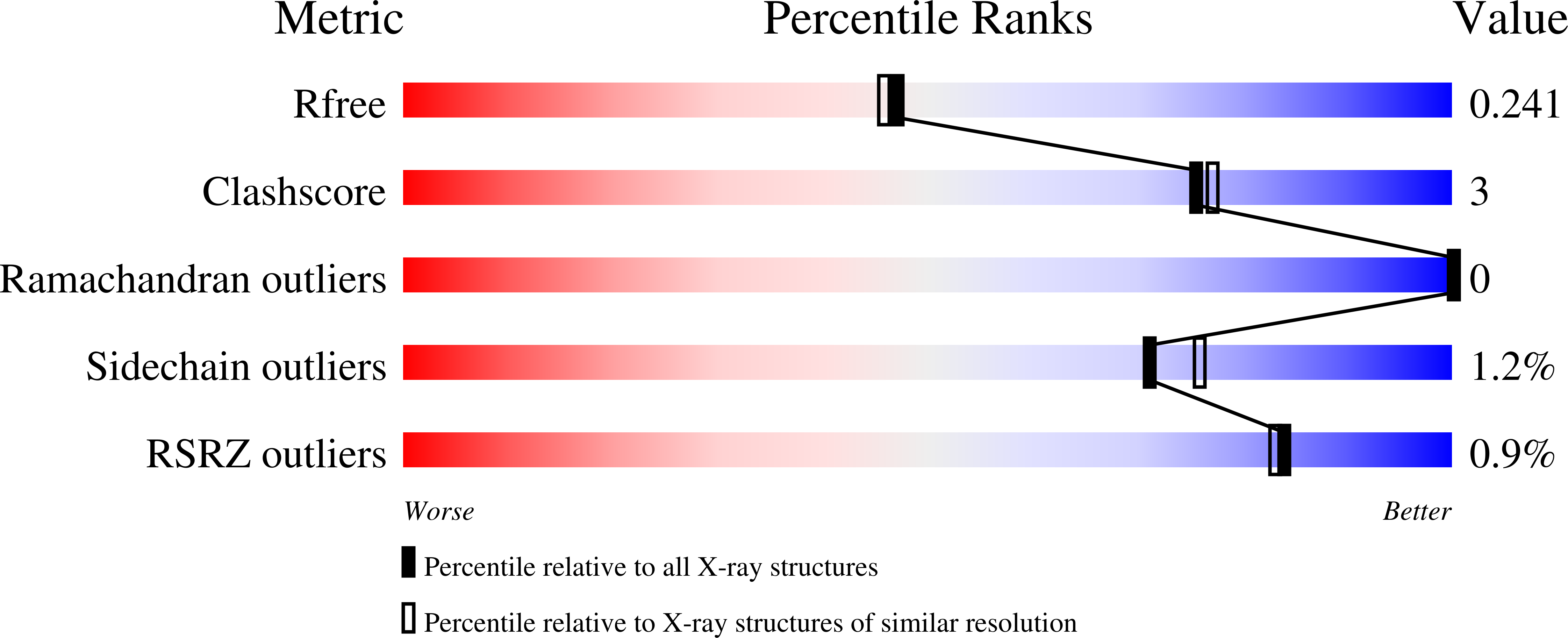
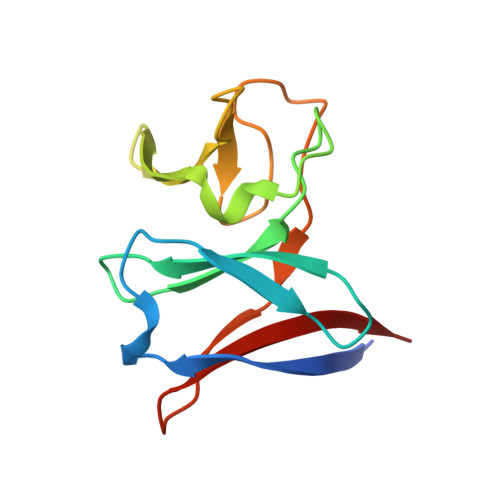
Crystal Structure of Nird, the Small Subunit of the Nitrite Reductase Nirbd from Mycobacterium Tuberculosis, at 2.0 Angstrom Resolution
Izumi, A., Schnell, R., Schneider, G.(2012) Proteins 80: 2799
- PubMed: 22965870
- DOI: https://doi.org/10.1002/prot.24177
- Primary Citation of Related Structures:
4AIV - PubMed Abstract:
NirD is part of the nitrite reductase complex NirBD that catalyses the reduction of nitrite to NH(3) in nitrate assimilation and anaerobic respiration. The crystal structure analysis of NirD from Mycobacterium tuberculosis shows a double β-sandwich fold. NirD is related in three-dimensional structure and sequence to the Rieske proteins; however, it does not contain any Fe-S cluster or other cofactors that might be involved in electron transfer. A cysteine residue at the protein surface, conserved in NirD homologues lacking the iron-sulfur cluster might be important for the interaction with NirB and possibly stabilize one of the Fe-S centers in this subunit.
Organizational Affiliation:
Department of Medical Biochemistry and Biophysics, Karolinska Institutet, S-17177 Stockholm, Sweden.