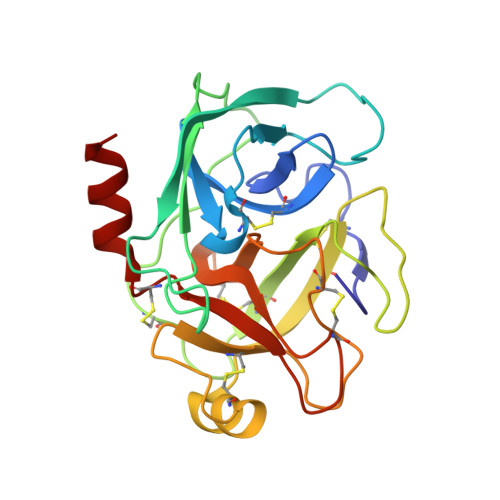
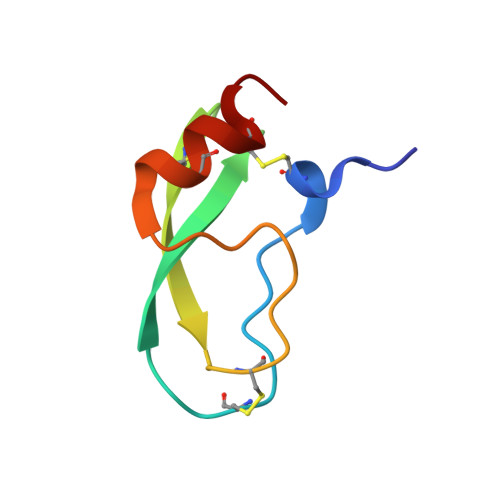
Fluorine teams up with water to restore inhibitor activity to mutant BPTI.
Ye, S., Loll, B., Berger, A.A., Mulow, U., Alings, C., Wahl, M.C., Koksch, B.(2015) Chem Sci 6: 5246-5254
- PubMed: 29449928
- DOI: https://doi.org/10.1039/c4sc03227f
- Primary Citation of Related Structures:
4Y0Y, 4Y0Z, 4Y10, 4Y11 - PubMed Abstract:
Introducing fluorine into molecules has a wide range of effects on their physicochemical properties, often desirable but in most cases unpredictable. The fluorine atom imparts the C-F bond with low polarizability and high polarity, and significantly affects the behavior of neighboring functional groups, in a covalent or noncovalent manner. Here, we report that fluorine, present in the form of a single fluoroalkyl amino acid side chain in the P1 position of the well-characterized serine-protease inhibitor BPTI, can fully restore inhibitor activity to a mutant that contains the corresponding hydrocarbon side chain at the same site. High resolution crystal structures were obtained for four BPTI variants in complex with bovine β-trypsin, revealing changes in the stoichiometry and dynamics of water molecules in the S1 subsite. These results demonstrate that the introduction of fluorine into a protein environment can result in "chemical complementation" that has a significantly favorable impact on protein-protein interactions.
Organizational Affiliation:
Department of Biology, Chemistry, and Pharmacy , Freie Universität Berlin , Institute of Chemistry and Biochemistry , Takustr. 3 , Berlin, 14195 , Germany . Email: beate.koksch@fu-berlin.de ; ; Tel: +49-30-83855344.