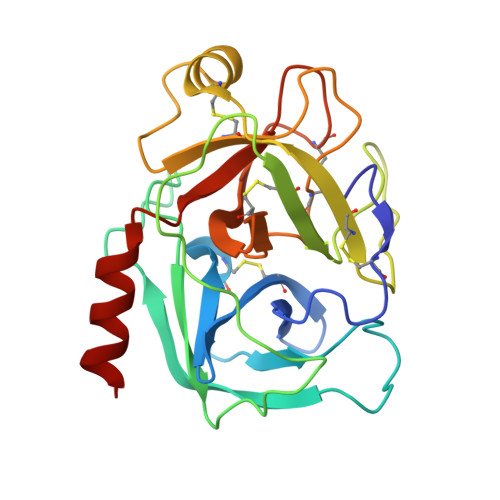
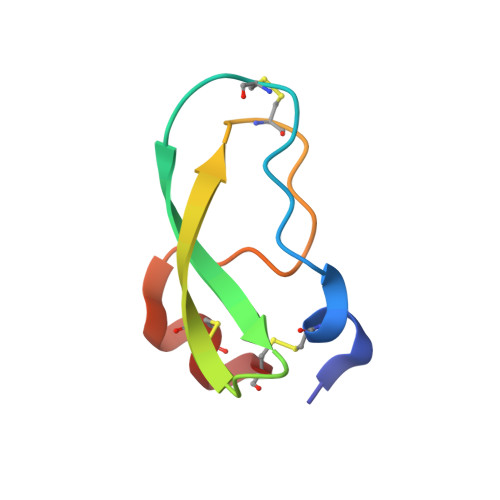
Mesotrypsin Has Evolved Four Unique Residues to Cleave Trypsin Inhibitors as Substrates.
Alloy, A.P., Kayode, O., Wang, R., Hockla, A., Soares, A.S., Radisky, E.S.(2015) J Biol Chem 290: 21523-21535
- PubMed: 26175157
- DOI: https://doi.org/10.1074/jbc.M115.662429
- Primary Citation of Related Structures:
4WWY, 4WXV - PubMed Abstract:
Human mesotrypsin is highly homologous to other mammalian trypsins, and yet it is functionally unique in possessing resistance to inhibition by canonical serine protease inhibitors and in cleaving these inhibitors as preferred substrates. Arg-193 and Ser-39 have been identified as contributors to the inhibitor resistance and cleavage capability of mesotrypsin, but it is not known whether these residues fully account for the unusual properties of mesotrypsin. Here, we use human cationic trypsin as a template for engineering a gain of catalytic function, assessing mutants containing mesotrypsin-like mutations for resistance to inhibition by bovine pancreatic trypsin inhibitor (BPTI) and amyloid precursor protein Kunitz protease inhibitor (APPI), and for the ability to hydrolyze these inhibitors as substrates. We find that Arg-193 and Ser-39 are sufficient to confer mesotrypsin-like resistance to inhibition; however, compared with mesotrypsin, the trypsin-Y39S/G193R double mutant remains 10-fold slower at hydrolyzing BPTI and 2.5-fold slower at hydrolyzing APPI. We identify two additional residues in mesotrypsin, Lys-74 and Asp-97, which in concert with Arg-193 and Ser-39 confer the full catalytic capability of mesotrypsin for proteolysis of BPTI and APPI. Novel crystal structures of trypsin mutants in complex with BPTI suggest that these four residues function cooperatively to favor conformational dynamics that assist in dissociation of cleaved inhibitors. Our results reveal that efficient inhibitor cleavage is a complex capability to which at least four spatially separated residues of mesotrypsin contribute. These findings suggest that inhibitor cleavage represents a functional adaptation of mesotrypsin that may have evolved in response to positive selection pressure.
Organizational Affiliation:
From the Department of Cancer Biology, Mayo Clinic Comprehensive Cancer Center, Jacksonville, Florida 32224 and.