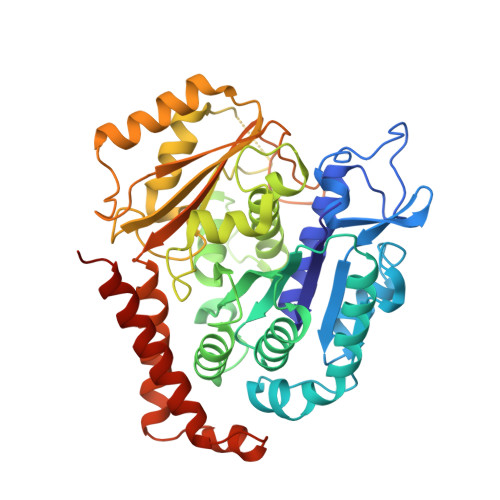
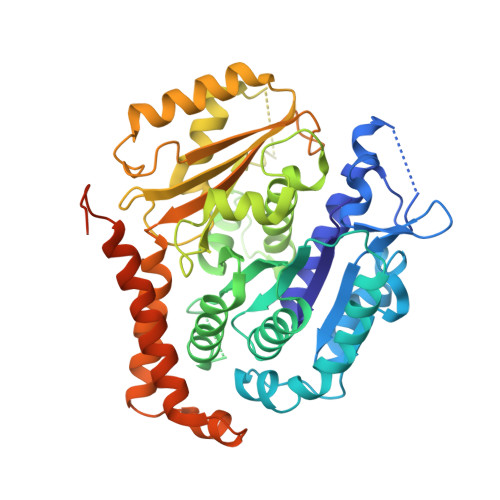
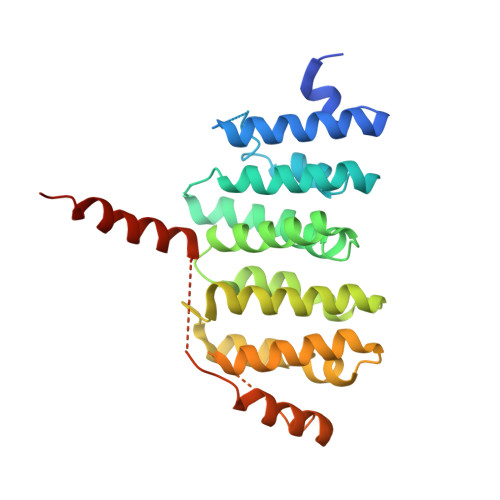
A TOG: alpha beta-tubulin complex structure reveals conformation-based mechanisms for a microtubule polymerase.
Ayaz, P., Ye, X., Huddleston, P., Brautigam, C.A., Rice, L.M.(2012) Science 337: 857-860
- PubMed: 22904013
- DOI: https://doi.org/10.1126/science.1221698
- Primary Citation of Related Structures:
4FFB - PubMed Abstract:
Stu2p/XMAP215/Dis1 family proteins are evolutionarily conserved regulatory factors that use αβ-tubulin-interacting tumor overexpressed gene (TOG) domains to catalyze fast microtubule growth. Catalysis requires that these polymerases discriminate between unpolymerized and polymerized forms of αβ-tubulin, but the mechanism by which they do so has remained unclear. Here, we report the structure of the TOG1 domain from Stu2p bound to yeast αβ-tubulin. TOG1 binds αβ-tubulin in a way that excludes equivalent binding of a second TOG domain. Furthermore, TOG1 preferentially binds a curved conformation of αβ-tubulin that cannot be incorporated into microtubules, contacting α- and β-tubulin surfaces that do not participate in microtubule assembly. Conformation-selective interactions with αβ-tubulin explain how TOG-containing polymerases discriminate between unpolymerized and polymerized forms of αβ-tubulin and how they selectively recognize the growing end of the microtubule.
Organizational Affiliation:
Department of Biophysics, University of Texas Southwestern Medical Center, 5323 Harry Hines Blvd, Dallas, TX 75390, USA.