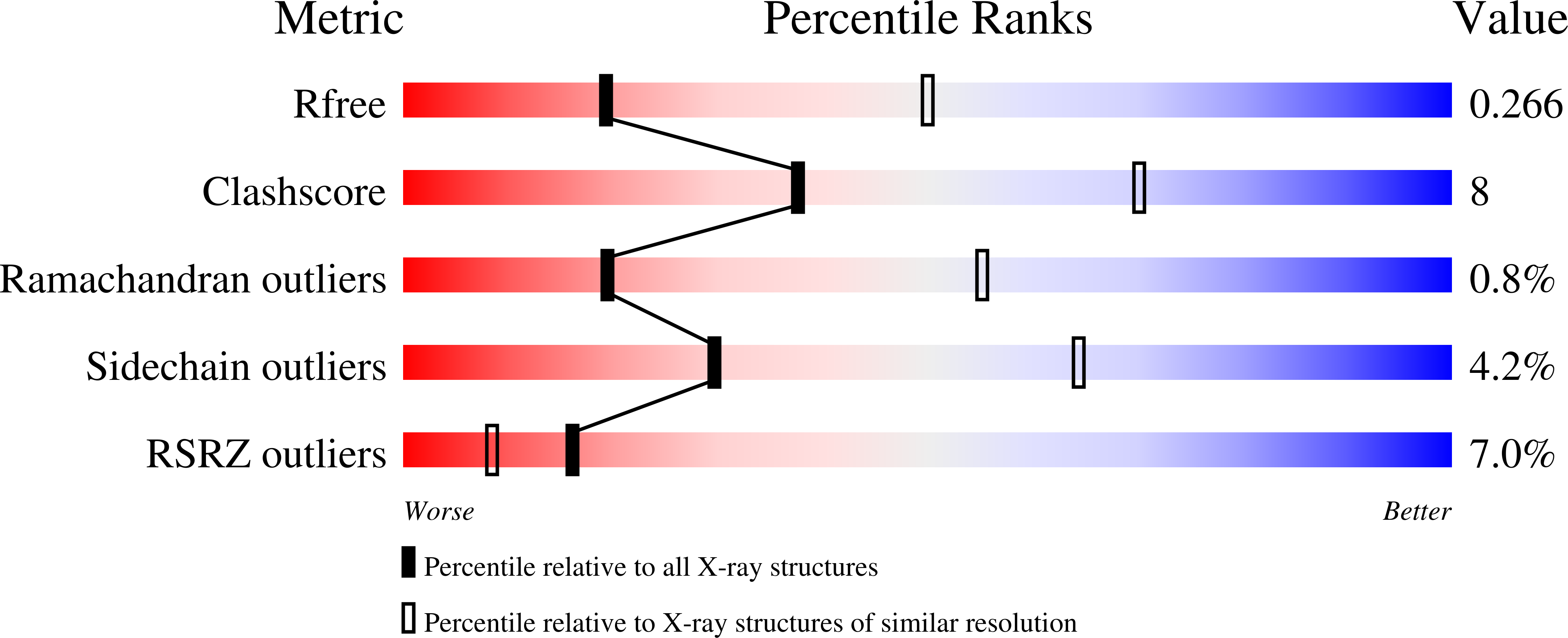
A two-domain elevator mechanism for sodium/proton antiport.
Lee, C., Kang, H.J., von Ballmoos, C., Newstead, S., Uzdavinys, P., Dotson, D.L., Iwata, S., Beckstein, O., Cameron, A.D., Drew, D.(2013) Nature 501: 573-577
- PubMed: 23995679
- DOI: https://doi.org/10.1038/nature12484
- Primary Citation of Related Structures:
4BWZ - PubMed Abstract:
Sodium/proton (Na(+)/H(+)) antiporters, located at the plasma membrane in every cell, are vital for cell homeostasis. In humans, their dysfunction has been linked to diseases, such as hypertension, heart failure and epilepsy, and they are well-established drug targets. The best understood model system for Na(+)/H(+) antiport is NhaA from Escherichia coli, for which both electron microscopy and crystal structures are available. NhaA is made up of two distinct domains: a core domain and a dimerization domain. In the NhaA crystal structure a cavity is located between the two domains, providing access to the ion-binding site from the inward-facing surface of the protein. Like many Na(+)/H(+) antiporters, the activity of NhaA is regulated by pH, only becoming active above pH 6.5, at which point a conformational change is thought to occur. The only reported NhaA crystal structure so far is of the low pH inactivated form. Here we describe the active-state structure of a Na(+)/H(+) antiporter, NapA from Thermus thermophilus, at 3 Å resolution, solved from crystals grown at pH 7.8. In the NapA structure, the core and dimerization domains are in different positions to those seen in NhaA, and a negatively charged cavity has now opened to the outside. The extracellular cavity allows access to a strictly conserved aspartate residue thought to coordinate ion binding directly, a role supported here by molecular dynamics simulations. To alternate access to this ion-binding site, however, requires a surprisingly large rotation of the core domain, some 20° against the dimerization interface. We conclude that despite their fast transport rates of up to 1,500 ions per second, Na(+)/H(+) antiporters operate by a two-domain rocking bundle model, revealing themes relevant to secondary-active transporters in general.
Organizational Affiliation:
Division of Molecular Biosciences, Imperial College London, London SW7 2AZ, UK.