Crystal Structure of Kiwi-Fruit Allergen Act D 2
Pavkov-Keller, T., Bublin, M., Jankovic, M., Breiteneder, H., Keller, W.To be published.
Experimental Data Snapshot
wwPDB Validation 3D Report Full Report
Entity ID: 1 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| THAUMATIN-LIKE PROTEIN | 201 | Actinidia deliciosa | Mutation(s): 0 | 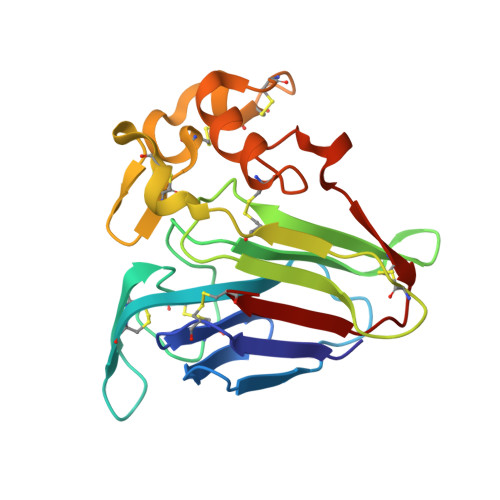 | |
UniProt | |||||
Find proteins for P81370 (Actinidia deliciosa) Explore P81370 Go to UniProtKB: P81370 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | P81370 | ||||
Sequence AnnotationsExpand | |||||
| |||||
| Ligands 1 Unique | |||||
|---|---|---|---|---|---|
| ID | Chains | Name / Formula / InChI Key | 2D Diagram | 3D Interactions | |
| EPE Query on EPE | B [auth A] | 4-(2-HYDROXYETHYL)-1-PIPERAZINE ETHANESULFONIC ACID C8 H18 N2 O4 S JKMHFZQWWAIEOD-UHFFFAOYSA-N |  | ||
| Length ( Å ) | Angle ( ˚ ) |
|---|---|
| a = 71.541 | α = 90 |
| b = 48.312 | β = 100.72 |
| c = 50.58 | γ = 90 |
| Software Name | Purpose |
|---|---|
| REFMAC | refinement |
| DENZO | data reduction |
| SCALEPACK | data scaling |