Recombinant expression, molecular characterization and crystal structure of antitumor enzyme, l-lysine alpha-oxidase from Trichoderma viride.
Amano, M., Mizuguchi, H., Sano, T., Kondo, H., Shinyashiki, K., Inagaki, J., Tamura, T., Kawaguchi, T., Kusakabe, H., Imada, K., Inagaki, K.(2015) J Biochem 157: 549-559
- PubMed: 25648943
- DOI: https://doi.org/10.1093/jb/mvv012
- Primary Citation of Related Structures:
3X0V - PubMed Abstract:
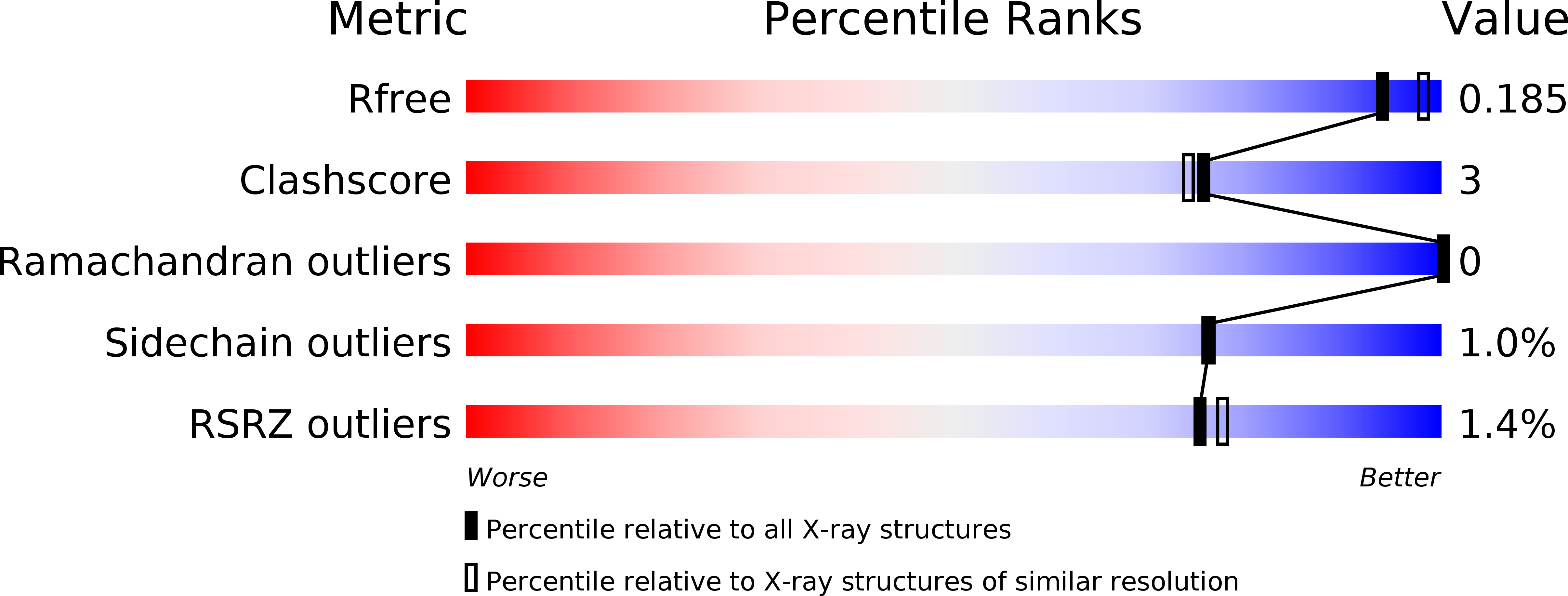
L-Lysine α-oxidase (LysOX) from Trichoderma viride is a homodimeric 112 kDa flavoenzyme that catalyzes the oxidative deamination of L-lysine to form α-keto-ε-aminocaproate. LysOX severely inhibited growth of cancer cells but showed relatively low cytotoxicity for normal cells. We have determined the cDNA nucleotide sequence encoding LysOX from T. viride. The full-length cDNA consists of 2,119 bp and encodes a possible signal peptide (Met1-Arg77) and the mature protein (Ala78-Ile617). The LysOX gene have been cloned and heterologously expressed in Streptomyces lividans TK24 with the enzyme activity up to 9.8 U/ml. The enzymatic properties of the purified recombinant LysOX, such as substrate specificity and thermal stability, are same as those of native LysOX. The crystal structure of LysOX at 1.9 Å resolution revealed that the overall structure is similar to that of snake venom L-amino acid oxidase (LAAO), and the residues involved in the interaction with the amino or carboxy group of the substrate are structurally conserved. However, the entrance and the inner surface structures of the funnel to the active site, as well as the residues involved in the substrate side-chain recognition, are distinct from LAAOs. These structural differences well explain the unique substrate specificity of LysOX.
Organizational Affiliation:
Department of Biofunctional Chemistry, Graduate School of Environmental and Life Science, Okayama University, Okayama 700-8530, Japan; Department of Macromolecular Science, Graduate School of Science, Osaka University, Toyonaka, Osaka 560-0043, Japan; Graduate School of Medicine, Dentistry and Pharmaceutical Sciences, Okayama University, Okayama 700-8558, Japan; and Enzyme Sensor Co., Ltd., Tsukuba, Ibaraki 305-0047, Japan.