Flexibility and multiple conformations of the activation and glycine rich loops of aurora A accompanying inhibitor binding
Oliveira, T.M., Kairies, N., Engh, R.A.To be published.
Experimental Data Snapshot
Entity ID: 1 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Aurora kinase A | A, B [auth C], C [auth E], D [auth G] | 261 | Homo sapiens | Mutation(s): 0 Gene Names: AURKA, AIK, AIRK1, ARK1, AURA, AYK1, BTAK, IAK1, STK15, STK6 EC: 2.7.11.1 | 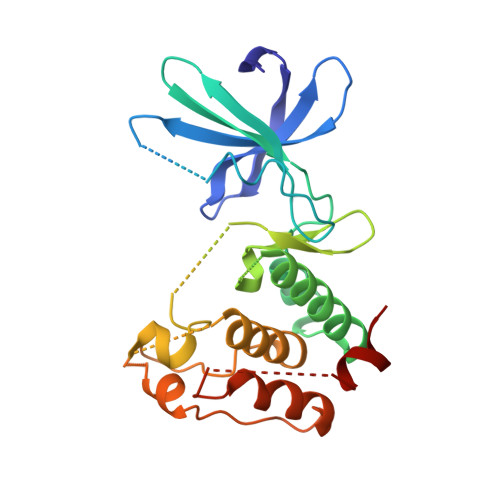 |
UniProt & NIH Common Fund Data Resources | |||||
Find proteins for O14965 (Homo sapiens) Explore O14965 Go to UniProtKB: O14965 | |||||
PHAROS: O14965 GTEx: ENSG00000087586 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | O14965 | ||||
Sequence AnnotationsExpand | |||||
| |||||
| Ligands 1 Unique | |||||
|---|---|---|---|---|---|
| ID | Chains | Name / Formula / InChI Key | 2D Diagram | 3D Interactions | |
| N15 Query on N15 | E [auth A], F [auth C], G [auth E], H [auth G] | 2-{4-[3-(1H-benzimidazol-2-yl)-1H-indazol-6-yl]-1H-pyrazol-1-yl}-N-(3-methylbutyl)acetamide C24 H25 N7 O MBGGBSBQBVZBHN-UHFFFAOYSA-N |  | ||
| Length ( Å ) | Angle ( ˚ ) |
|---|---|
| a = 69.21 | α = 90 |
| b = 86.05 | β = 89.83 |
| c = 85.96 | γ = 90 |
| Software Name | Purpose |
|---|---|
| DNA | data collection |
| PHASER | phasing |
| REFMAC | refinement |
| XDS | data reduction |
| SCALA | data scaling |