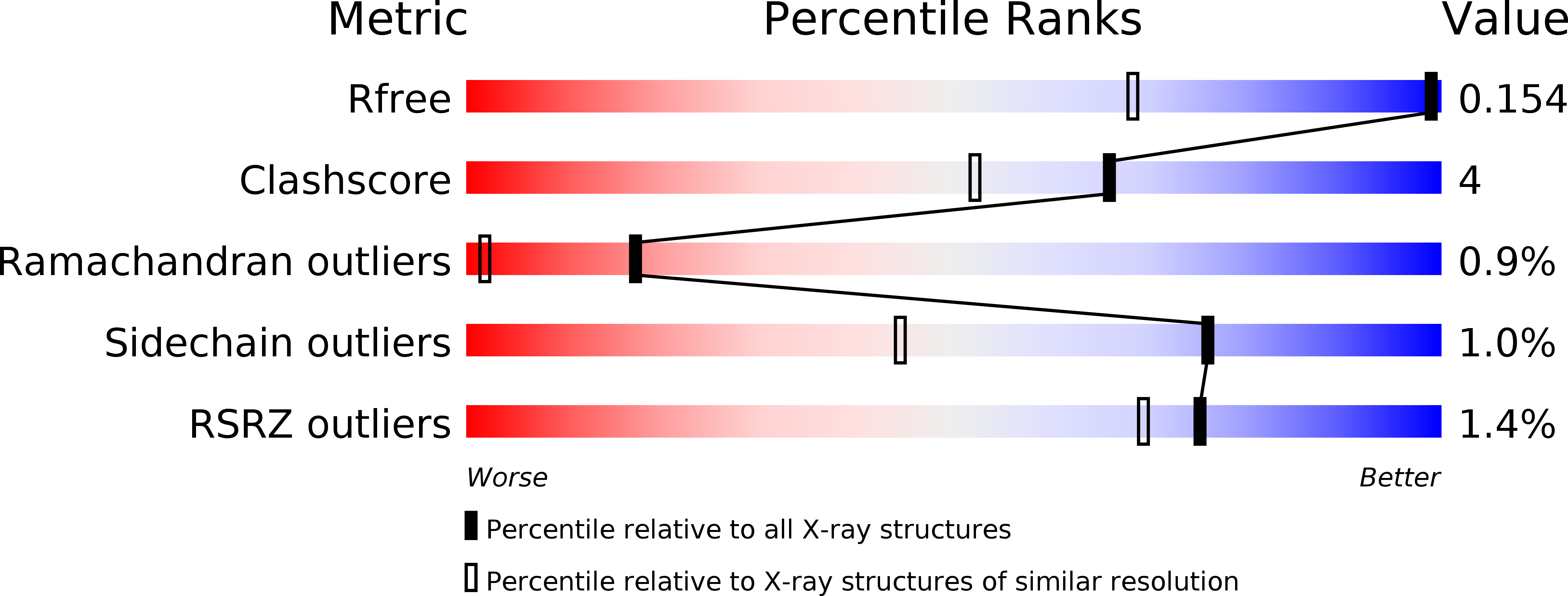
Structure of beta-1,4-mannanase from the common sea hare Aplysia kurodai at 1.05 A resolution.
Mizutani, K., Tsuchiya, S., Toyoda, M., Nanbu, Y., Tominaga, K., Yuasa, K., Takahashi, N., Tsuji, A., Mikami, B.(2012) Acta Crystallogr Sect F Struct Biol Cryst Commun 68: 1164-1168
- PubMed: 23027740
- DOI: https://doi.org/10.1107/S1744309112037074
- Primary Citation of Related Structures:
3VUP - PubMed Abstract:
β-1,4-Mannanase (EC 3.2.1.78) catalyzes the hydrolysis of β-1,4-glycosidic bonds within mannan, a major constituent group of the hemicelluloses. Bivalves and gastropods possess β-1,4-mannanase and may degrade mannan in seaweed and/or phytoplankton to obtain carbon and energy using the secreted enzymes in their digestive systems. In the present study, the crystal structure of AkMan, a gastropod β-1,4-mannanase prepared from the common sea hare Aplysia kurodai, was determined at 1.05 Å resolution. This is the first report of the three-dimensional structure of a gastropod β-1,4-mannanase. The structure was compared with bivalve β-1,4-mannanase and the roles of residues in the catalytic cleft were investigated. No obvious binding residue was found in subsite +1 and the substrate-binding site was exposed to the molecular surface, which may account for the enzymatic properties of mannanases that can digest complex substrates such as glucomannan and branched mannan.
Organizational Affiliation:
Laboratory of Applied Structural Biology, Division of Applied Life Sciences, Graduate School of Agriculture, Kyoto University, Uji, Kyoto 611-0011, Japan.