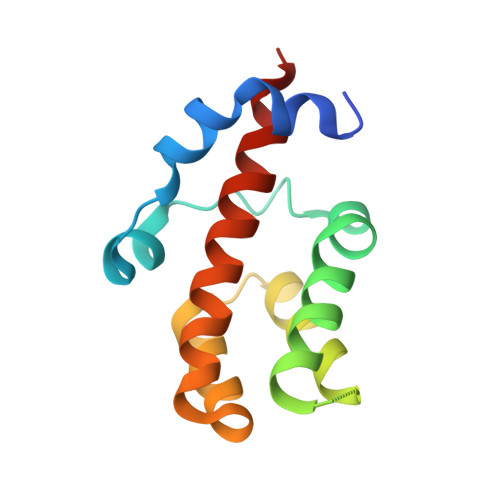
Crystal structure and functional characterization of the human RBM25 PWI domain and its flanking basic region
Gong, D.S., Yang, F., Li, F., Qian, D., Wu, M., Shao, Z., Wu, M., Wu, J., Shi, Y.(2013) Biochem J 450: 85-94
- PubMed: 23190262
- DOI: https://doi.org/10.1042/BJ20121382
- Primary Citation of Related Structures:
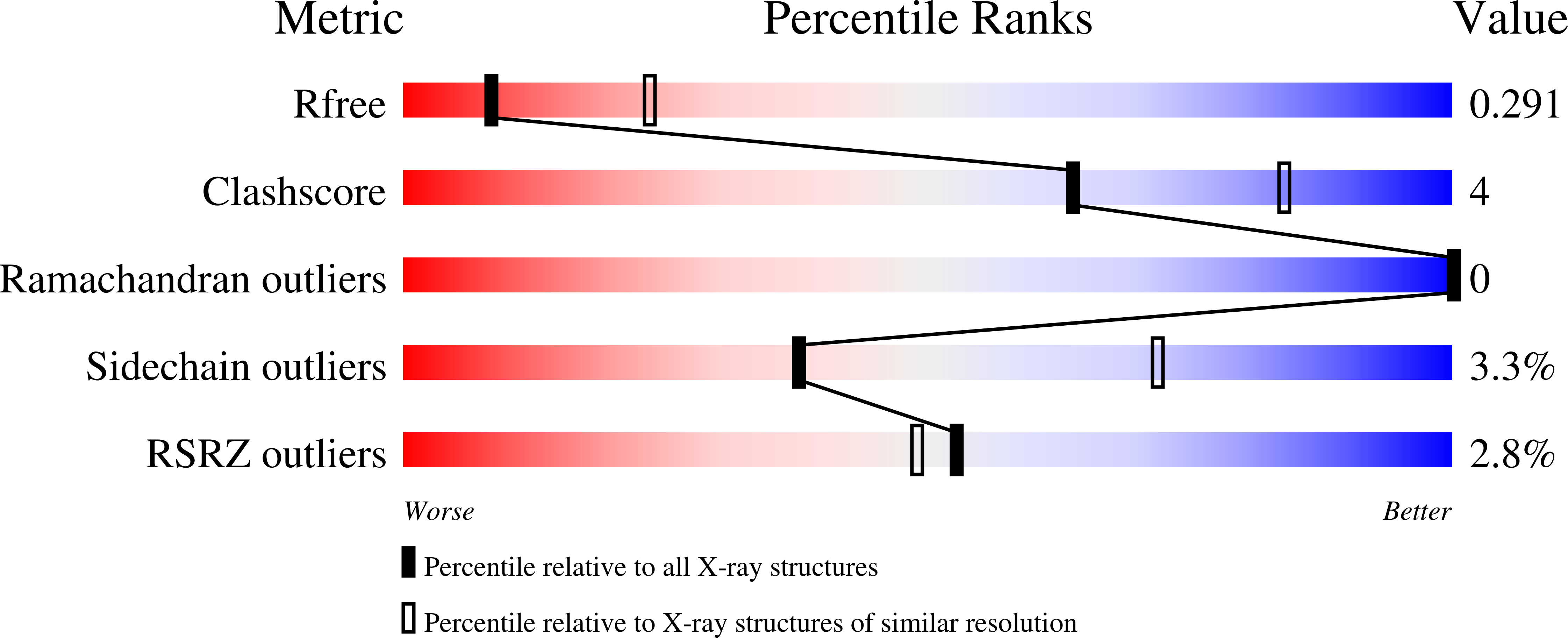
3V53 - PubMed Abstract:
Human RBM25 (RNA-binding motif protein 25) is a novel splicing factor that contains a PWI domain, a newly identified RNA/DNA-binding domain, and regulates Bcl-x pre-mRNA alternative splicing. The flanking basic region has been suggested to serve as a co-operative partner of the PWI domain in the binding of nucleic acids, but the structure of this basic region is unknown. In the present paper, we report the crystal structure of the RBM25 PWI domain and its flanking basic region. The PWI domain is revealed to comprise a conserved four-helix bundle, and the flanking basic region forms two α-helices and associates with helix H4 of the PWI domain. These interactions promote directly the formation of an enlarged nucleic-acid-binding platform. Structure-guided mutagenesis reveals a positively charged nucleic-acid-binding surface in the RBM25 PWI domain that is entirely different from that in the SRm160 PWI domain. Furthermore, we show that the promotion of the pro-apoptotic Bcl-xS isoform expression by RBM25 is facilitated by the PWI domain in vivo. Thus the present study suggests that the PWI domain plays an important role in the regulation of Bcl-x pre-mRNA alternative splicing.
Organizational Affiliation:
Hefei National Laboratory for Physical Sciences at the Microscale and School of Life Sciences, University of Science and Technology of China, Hefei, Anhui 230026, China.