DNA binding by the plant-specific NAC transcription factors in crystal and solution: a firm link to WRKY and GCM transcription factors.
Welner, D.H., Lindemose, S., Grossmann, J.G., Mollegaard, N.E., Olsen, A.N., Helgstrand, C., Skriver, K., Lo Leggio, L.(2012) Biochem J 444: 395-404
- PubMed: 22455904
- DOI: https://doi.org/10.1042/BJ20111742
- Primary Citation of Related Structures:
3SWM, 3SWP, 4DUL - PubMed Abstract:
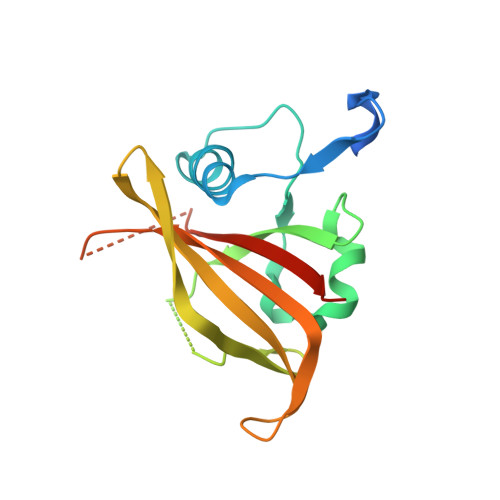
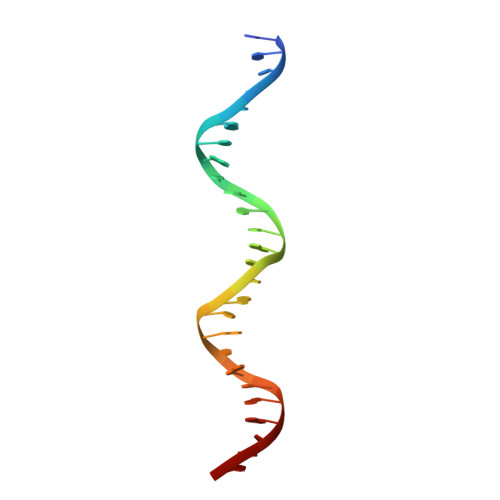
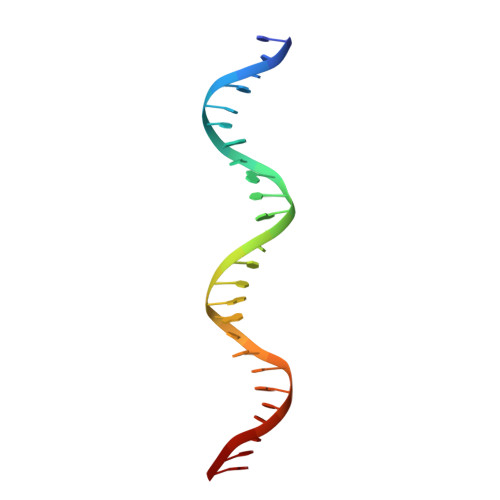
NAC (NAM/ATAF/CUC) plant transcription factors regulate essential processes in development, stress responses and nutrient distribution in important crop and model plants (rice, Populus, Arabidopsis), which makes them highly relevant in the context of crop optimization and bioenergy production. The structure of the DNA-binding NAC domain of ANAC019 has previously been determined by X-ray crystallography, revealing a dimeric and predominantly β-fold structure, but the mode of binding to cognate DNA has remained elusive. In the present study, information from low resolution X-ray structures and small angle X-ray scattering on complexes with oligonucleotides, mutagenesis and (DNase I and uranyl photo-) footprinting, is combined to form a structural view of DNA-binding, and for the first time provide experimental evidence for the speculated relationship between plant-specific NAC proteins, WRKY transcription factors and the mammalian GCM (Glial cell missing) transcription factors, which all use a β-strand motif for DNA-binding. The structure shows that the NAC domain inserts the edge of its core β-sheet into the major groove, while leaving the DNA largely undistorted. The structure of the NAC-DNA complex and a new crystal form of the unbound NAC also indicate limited flexibility of the NAC dimer arrangement, which could be important in recognizing suboptimal binding sites.
Organizational Affiliation:
Biophysical Chemistry Group, Department of Chemistry, University of Copenhagen, Universitetsparken 5, 2100 Copenhagen Ø, Denmark.