Single cystathionine beta-synthase domain-containing proteins modulate development by regulating the thioredoxin system in Arabidopsis
Yoo, K.S., Ok, S.H., Jeong, B.-C., Jung, K.W., Cui, M.H., Hyoung, S., Lee, M.-R., Song, H.K., Shin, J.S.(2011) Plant Cell 23: 3577-3594
- PubMed: 22021414
- DOI: https://doi.org/10.1105/tpc.111.089847
- Primary Citation of Related Structures:
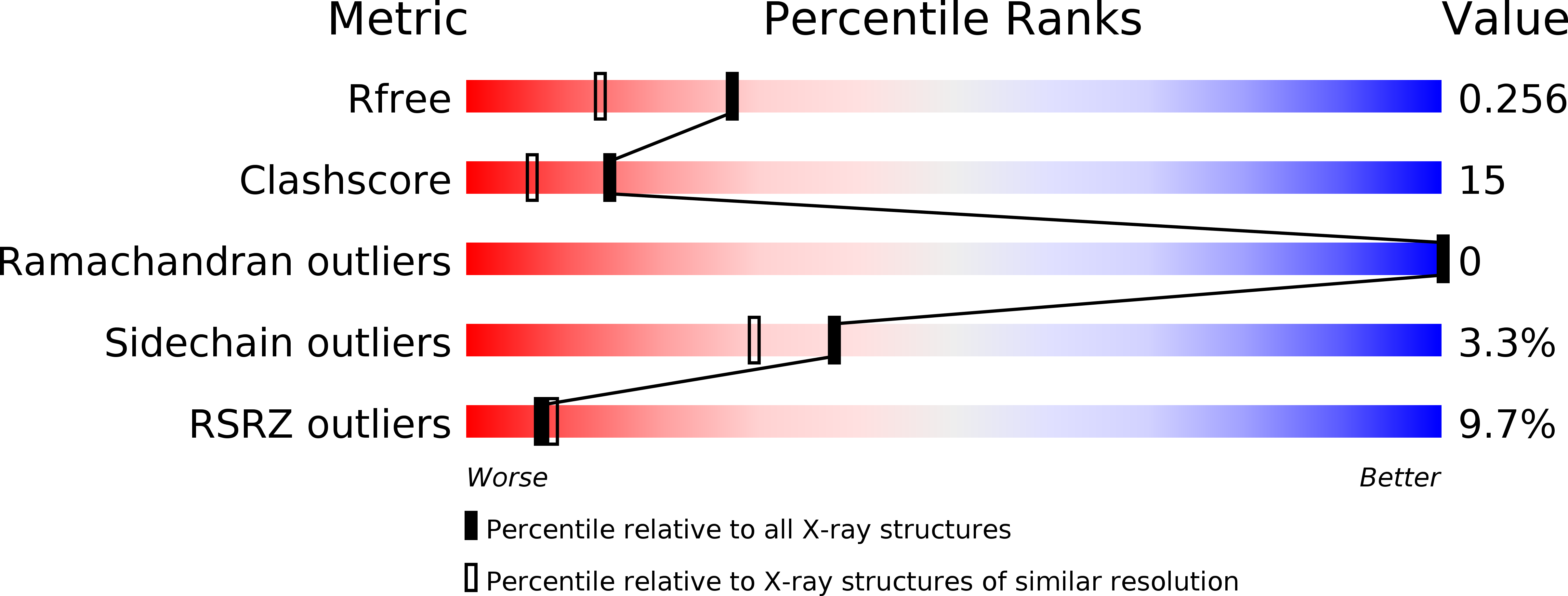
3SL7 - PubMed Abstract:
Plant thioredoxins (Trxs) participate in two redox systems found in different cellular compartments: the NADP-Trx system (NTS) in the cytosol and mitochondria and the ferredoxin-Trx system (FTS) in the chloroplast, where they function as redox regulators by regulating the activity of various target enzymes. The identities of the master regulators that maintain cellular homeostasis and modulate timed development through redox regulating systems have remained completely unknown. Here, we show that proteins consisting of a single cystathionine β-synthase (CBS) domain pair stabilize cellular redox homeostasis and modulate plant development via regulation of Trx systems by sensing changes in adenosine-containing ligands. We identified two CBS domain-containing proteins in Arabidopsis thaliana, CBSX1 and CBSX2, which are localized to the chloroplast, where they activate all four Trxs in the FTS. CBSX3 was found to regulate mitochondrial Trx members in the NTS. CBSX1 directly regulates Trxs and thereby controls H(2)O(2) levels and regulates lignin polymerization in the anther endothecium. It also affects plant growth by regulating photosynthesis-related [corrected] enzymes, such as malate dehydrogenase, via homeostatic regulation of Trxs. Based on our findings, we suggest that the CBSX proteins (or a CBS pair) are ubiquitous redox regulators that regulate Trxs in the FTS and NTS to modulate development and maintain homeostasis under conditions that are threatening to the cell.
Organizational Affiliation:
School of Life Sciences and Biotechnology, Korea University, Seoul 136-701, Korea. skysini@korea.ac.kr