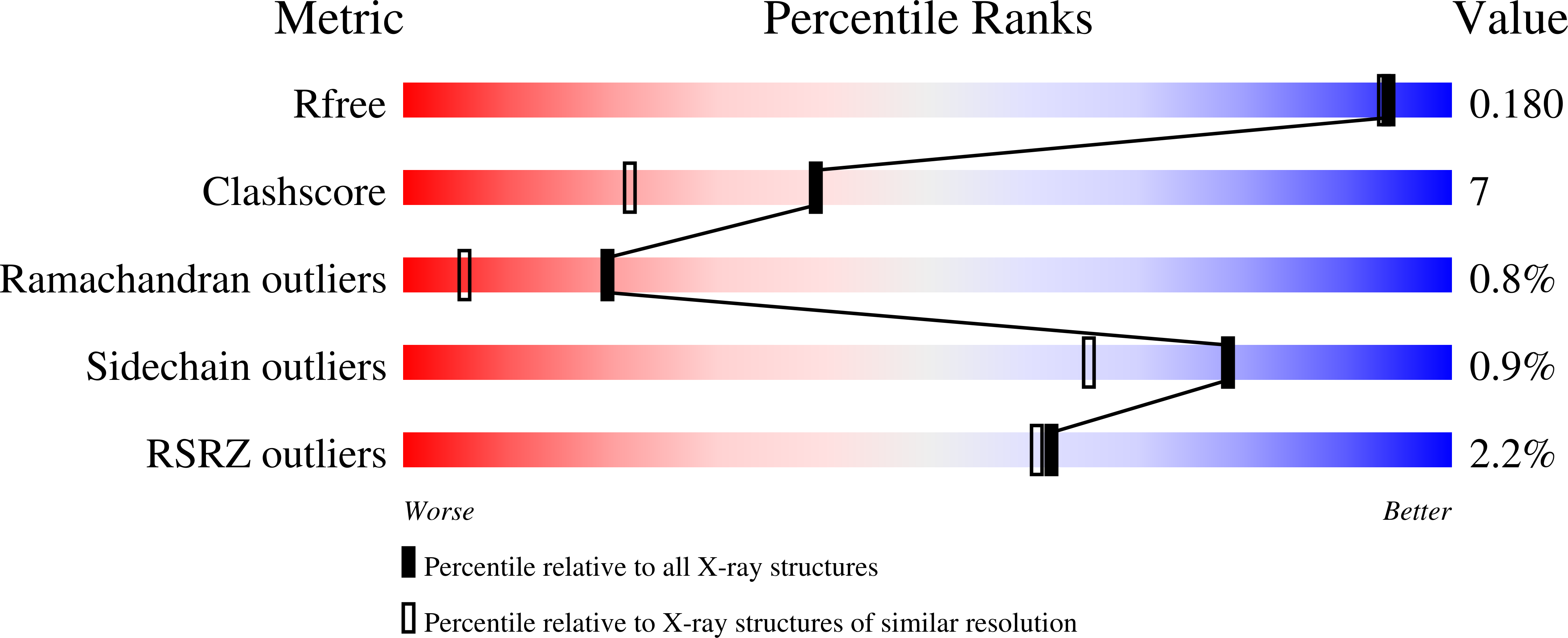
Structural and biochemical studies of serine acetyltransferase reveal why the parasite Entamoeba histolytica cannot form cysteine synthase complex
Kumar, S., Raj, I., Nagpal, I., Subbarao, N., Gourinath, S.(2011) J Biol Chem
- PubMed: 21297164
- DOI: https://doi.org/10.1074/jbc.M110.197376
- Primary Citation of Related Structures:
3P1B, 3P47, 3Q1X - PubMed Abstract:
Cysteine (Cys) plays a major role in growth and survival of the human parasite Entamoeba histolytica. We report here the crystal structure of serine acetyltransferase (SAT) isoform 1, a cysteine biosynthetic pathway enzyme from E. histolytica (EhSAT1) at 1.77 Å, in complex with its substrate serine (Ser) at 1.59 Å and inhibitor Cys at 1.78 Å resolution. EhSAT1 exists as a trimer both in solution as well as in crystal structure, unlike hexamers formed by other known SATs. The difference in oligomeric state is due to the N-terminal region of the EhSAT1, which has very low sequence similarity to known structures, also differs in orientation and charge distribution. The Ser and Cys bind to the same site, confirming that Cys is a competitive inhibitor of Ser. The disordered C-terminal region and the loop near the active site are responsible for solvent-accessible acetyl-CoA binding site and, thus, lose inhibition to acetyl-CoA by the feedback inhibitor Cys. Docking and fluorescence studies show that EhSAT1 C-terminal-mimicking peptides can bind to O-acetyl serine sulfhydrylase (EhOASS), whereas native C-terminal peptide does not show any binding. To test further, C-terminal end of EhSAT1 was mutated and found that it inhibits EhOASS, confirming modified EhSAT1 can bind to EhOASS. The apparent inability of EhSAT1 to form a hexamer and differences in the C-terminal region are likely to be the major reasons for the lack of formation of the large cysteine synthase complex and loss of a complex regulatory mechanism in E. histolytica.
Organizational Affiliation:
School of Life Sciences, Jawaharlal Nehru University, New Delhi, India.