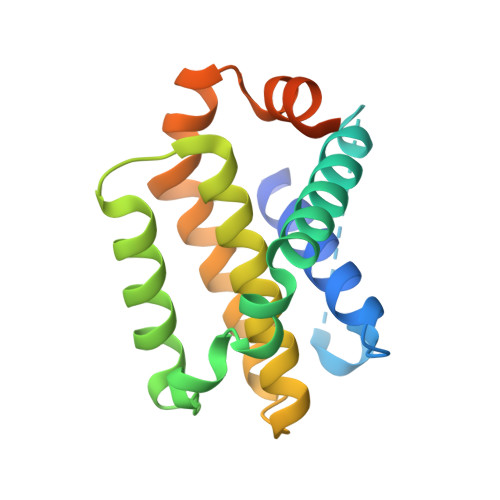
Mutation to Bax beyond the BH3 domain disrupts interactions with pro-survival proteins and promotes apoptosis
Czabotar, P.E., Lee, E.F., Thompson, G.V., Wardak, A.Z., Fairlie, W.D., Colman, P.M.(2011) J Biol Chem 286: 7123-7131
- PubMed: 21199865
- DOI: https://doi.org/10.1074/jbc.M110.161281
- Primary Citation of Related Structures:
3PK1, 3PL7 - PubMed Abstract:
Pro-survival members of the Bcl-2 family of proteins restrain the pro-apoptotic activity of Bax, either directly through interactions with Bax or indirectly by sequestration of activator BH3-only proteins, or both. Mutations in Bax that promote apoptosis can provide insight into how Bax is regulated. Here, we describe crystal structures of the pro-survival proteins Mcl-1 and Bcl-x(L) in complex with a 34-mer peptide from Bax that encompasses its BH3 domain. These structures reveal canonical interactions between four signature hydrophobic amino acids from the BaxBH3 domain and the BH3-binding groove of the pro-survival proteins. In both structures, Met-74 from the Bax peptide engages with the BH3-binding groove in a fifth hydrophobic interaction. Various Bax Met-74 mutants disrupt interactions between Bax and all pro-survival proteins, but these Bax mutants retain pro-apoptotic activity. Bax/Bak-deficient mouse embryonic fibroblast cells reconstituted with several Bax Met-74 mutants are more sensitive to the BH3 mimetic compound ABT-737 as compared with cells expressing wild-type Bax. Furthermore, the cells expressing Bax Met-74 mutants are less viable in colony assays even in the absence of an external apoptotic stimulus. These results support a model in which direct restraint of Bax by pro-survival Bcl-2 proteins is a barrier to apoptosis.
Organizational Affiliation:
Walter and Eliza Hall Institute of Medical Research, Parkville, Victoria 3052, Australia. czabotar@wehi.edu.au