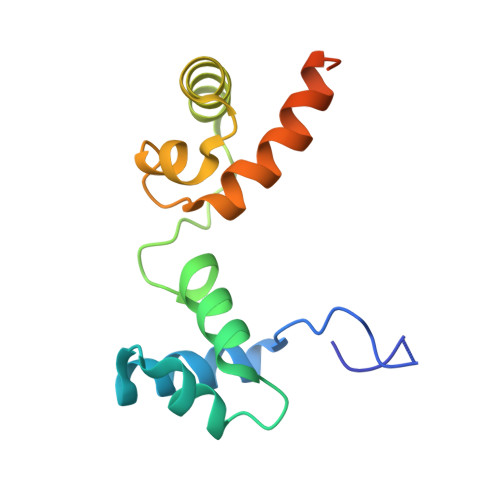
Molecular basis of the recognition of the ap65-1 gene transcription promoter elements by a Myb protein from the protozoan parasite Trichomonas vaginalis.
Jiang, I., Tsai, C.K., Chen, S.C., Wang, S.H., Amiraslanov, I., Chang, C.F., Wu, W.J., Tai, J.H., Liaw, Y.C., Huang, T.H.(2011) Nucleic Acids Res 39: 8992-9008
- PubMed: 21771861
- DOI: https://doi.org/10.1093/nar/gkr558
- Primary Citation of Related Structures:
3OSF, 3OSG - PubMed Abstract:
Iron-inducible transcription of the ap65-1 gene in Trichomonas vaginalis involves at least three Myb-like transcriptional factors (tvMyb1, tvMyb2 and tvMyb3) that differentially bind to two closely spaced promoter sites, MRE-1/MRE-2r and MRE-2f. Here, we defined a fragment of tvMyb2 comprising residues 40-156 (tvMyb2₄₀₋₁₅₆) as the minimum structural unit that retains near full binding affinity with the promoter DNAs. Like c-Myb in vertebrates, the DNA-free tvMyb2₄₀₋₁₅₆ has a flexible and open conformation. Upon binding to the promoter DNA elements, tvMyb2₄₀₋₁₅₆ undergoes significant conformational re-arrangement and structure stabilization. Crystal structures of tvMyb2₄₀₋₁₅₆ in complex with promoter element-containing DNA oligomers showed that 5'-a/gACGAT-3' is the specific base sequence recognized by tvMyb2₄₀₋₁₅₆, which does not fully conform to that of the Myb binding site sequence. Furthermore, Lys⁴⁹, which is upstream of the R2 motif (amino acids 52-102) also participates in specific DNA sequence recognition. Intriguingly, tvMyb2₄₀₋₁₅₆ binds to the promoter elements in an orientation opposite to that proposed in the HADDOCK model of the tvMyb1₃₅₋₁₄₁/MRE-1-MRE-2r complex. These results shed new light on understanding the molecular mechanism of Myb-DNA recognition and provide a framework to study the molecular basis of transcriptional regulation of myriad Mybs in T. vaginalis.
Organizational Affiliation:
Institute of Biomedical Sciences, Academia Sinica, Taipei 115, Taiwan, ROC.