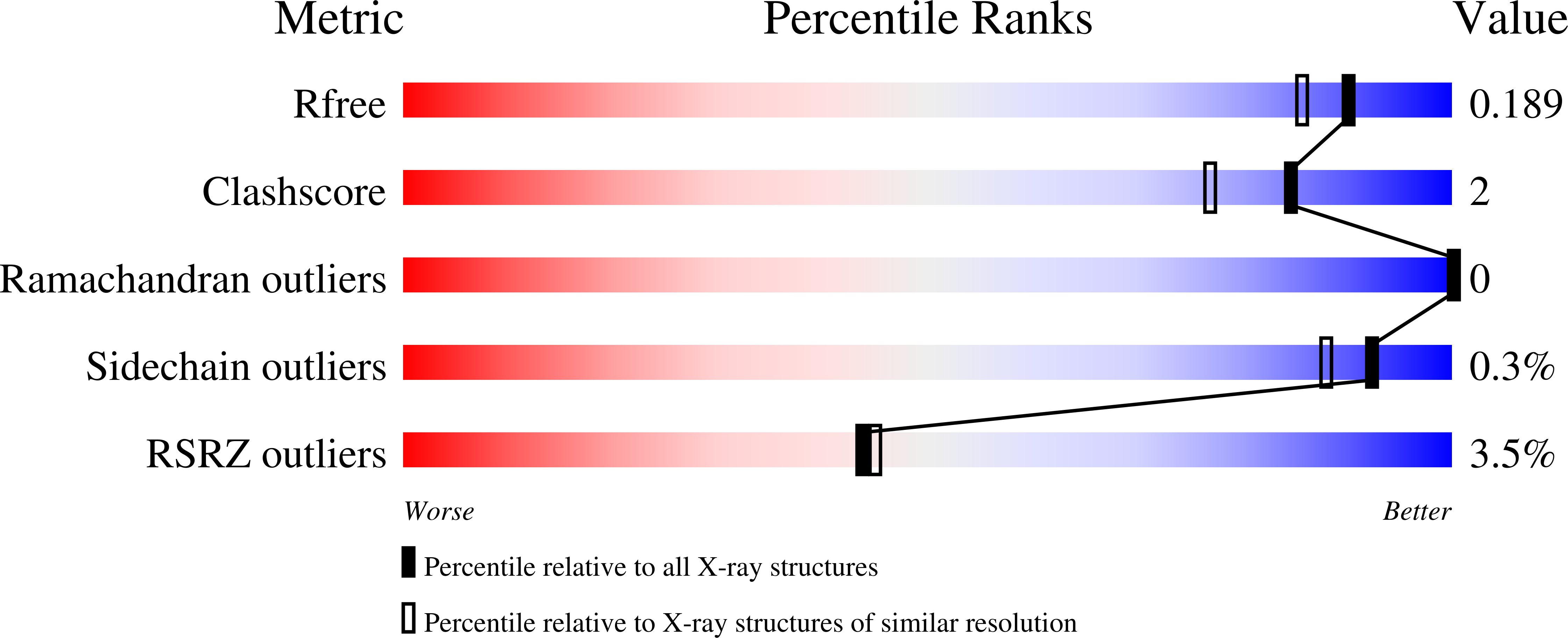
Structural analysis of the receptor binding domain of botulinum neurotoxin serotype D.
Zhang, Y., Buchko, G.W., Qin, L., Robinson, H., Varnum, S.M.(2010) Biochem Biophys Res Commun 401: 498-503
- PubMed: 20858456
- DOI: https://doi.org/10.1016/j.bbrc.2010.09.063
- Primary Citation of Related Structures:
3OGG - PubMed Abstract:
Botulinum neurotoxins (BoNTs) are the most toxic proteins known. The mechanism for entry into neuronal cells for serotypes A, B, E, F, and G involves a well understood dual receptor (protein and ganglioside) process, however, the mechanism of entry for serotypes C and D remains unclear. To provide structural insights into how BoNT/D enters neuronal cells, the crystal structure of the receptor binding domain (S863-E1276) for this serotype (BoNT/D-HCR) was determined at 1.65Å resolution. While BoNT/D-HCR adopts an overall fold similar to that observed in other known BoNT HCRs, several major structural differences are present. These structural differences are located at, or near, putative receptor binding sites and may be responsible for BoNT/D host preferences. Two loops, S1195-I1204 and K1236-N1244, located on both sides of the putative protein receptor binding pocket, are displaced >10Å relative to the corresponding residues in the crystal structures of BoNT/B and G. Obvious clashes were observed in the putative protein receptor binding site when the BoNT/B protein receptor synaptotagmin II was modeled into the BoNT/D-HCR structure. Although a ganglioside binding site has never been unambiguously identified in BoNT/D-HCR, a shallow cavity in an analogous location to the other BoNT serotypes HCR domains is observed in BoNT/D-HCR that has features compatible with membrane binding. A portion of a loop near the putative receptor binding site, K1236-N1244, is hydrophobic and solvent-exposed and may directly bind membrane lipids. Liposome-binding experiments with BoNT/D-HCR demonstrate that this membrane lipid may be phosphatidylethanolamine.
Organizational Affiliation:
Cell Biology and Biochemistry Group, Biological Sciences Division, Pacific Northwest National Laboratory, Richland, WA 99352, USA.