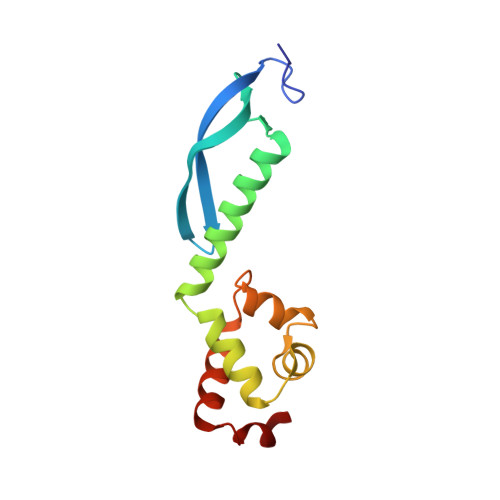
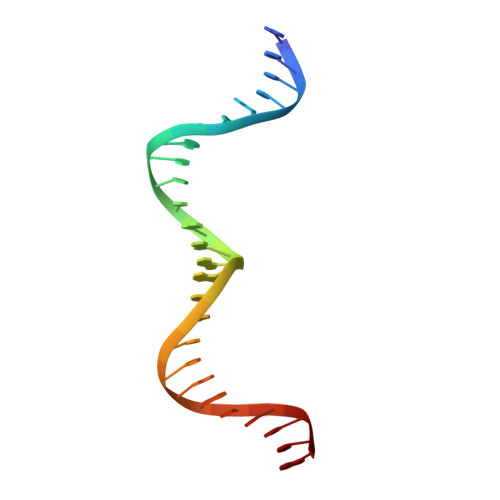
Structure of the Escherichia coli Antitoxin MqsA (YgiT/b3021) Bound to Its Gene Promoter Reveals Extensive Domain Rearrangements and the Specificity of Transcriptional Regulation.
Brown, B.L., Wood, T.K., Peti, W., Page, R.(2011) J Biol Chem 286: 2285-2296
- PubMed: 21068382
- DOI: https://doi.org/10.1074/jbc.M110.172643
- Primary Citation of Related Structures:
3O9X - PubMed Abstract:
Bacterial cultures, especially biofilms, produce a small number of persister cells, a genetically identical subpopulation of wild type cells that are metabolically dormant, exhibit multidrug tolerance, and are highly enriched in bacterial toxins. The gene most highly up-regulated in Escherichia coli persisters is mqsR, a ribonuclease toxin that, along with mqsA, forms a novel toxin·antitoxin (TA) system. Like all known TA systems, both the MqsR·MqsA complex and MqsA alone regulate their own transcription. Despite the importance of TA systems in persistence and biofilms, very little is known about how TA modules, and antitoxins in particular, bind and recognize DNA at a molecular level. Here, we report the crystal structure of MqsA bound to a 26-bp fragment from the mqsRA promoter. We show that MqsA binds DNA predominantly via its C-terminal helix-turn-helix domain, with direct binding of recognition helix residues Asn(97) and Arg(101) to the DNA major groove. Unexpectedly, the structure also revealed that the MqsA N-terminal domain interacts with the DNA phosphate backbone. This results in a more than 105° rotation of the N-terminal domains between the free and complexed states, an unprecedented rearrangement for an antitoxin. The structure also shows that MqsA bends the DNA by more than 55° in order to achieve symmetrical binding. Finally, using a combination of biochemical and NMR studies, we show that the DNA sequence specificity of MqsA is mediated by direct readout.
Organizational Affiliation:
Department of Molecular Pharmacology, Physiology, and Biotechnology, Brown University, Providence, Rhode Island 02912, USA.