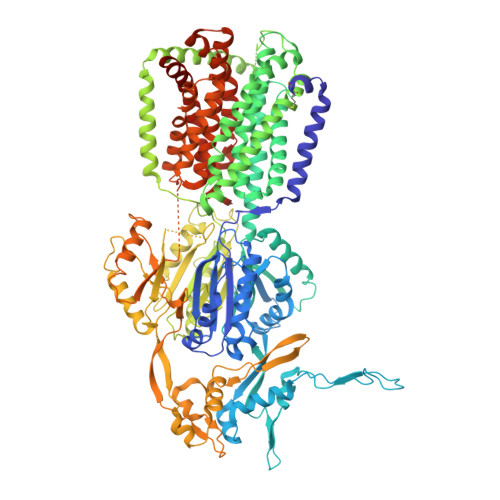
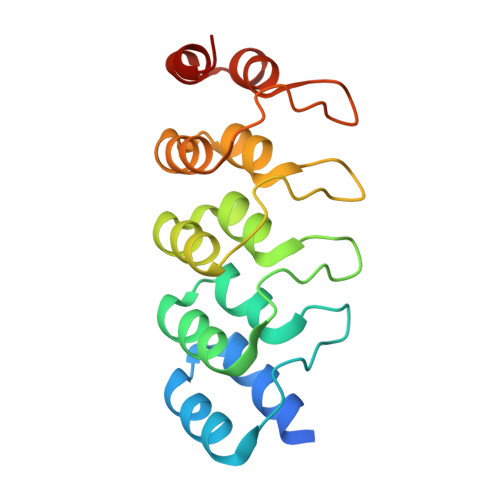
Designed ankyrin repeat protein binders for the crystallization of AcrB: Plasticity of the dominant interface
Monroe, N., Sennhauser, G., Seeger, M.A., Briand, C., Grutter, M.G.(2011) J Struct Biol 174: 269-281
- PubMed: 21296164
- DOI: https://doi.org/10.1016/j.jsb.2011.01.014
- Primary Citation of Related Structures:
3NOC, 3NOG - PubMed Abstract:
The formation of well-diffracting crystals is a major bottleneck in structural analysis of membrane proteins by X-ray crystallography. One approach to improve crystal quality is the use of DARPins as crystallization chaperones. Here, we present a detailed analysis of the interaction between DARPins and the integral membrane protein AcrB. We find that binders selected in vitro by ribosome display share a common epitope. The comparative analysis of three crystal structures of AcrB-DARPin complexes allowed us to study the plasticity of the interaction with this dominant binding site. Seemingly redundant AcrB-DARPin crystals show substantially different diffraction quality as a result of subtle differences in the binding geometry. This work exemplifies the importance to screen a number of crystallization chaperones to obtain optimal diffraction data. Crystallographic analysis is complemented by biophysical characterization of nine AcrB binders. We observe that small variations in the interface can lead to differing behavior of the DARPins with regards to affinity, stoichiometry of the complexes and specificity for their target.
Organizational Affiliation:
Department of Biochemistry, University of Zurich, Winterthurerstrasse 190, 8057 Zurich, Switzerland. nmonroe@bioc.uzh.ch