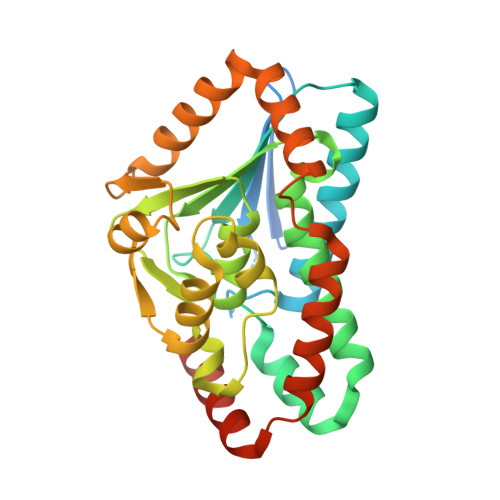
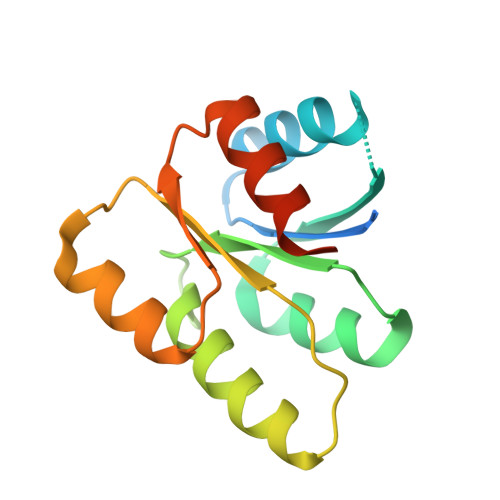
Structural Basis of the Sensor-Synthase Interaction in Autoinduction of the Quorum Sensing Signal DSF Biosynthesis
Cheng, Z., He, Y.W., Lim, S.C., Qamra, R., Walsh, M.A., Zhang, L.H., Song, H.(2010) Structure 18: 1199-1209
- PubMed: 20826346
- DOI: https://doi.org/10.1016/j.str.2010.06.011
- Primary Citation of Related Structures:
3M6M, 3M6N - PubMed Abstract:
The diffusible signal factor (DSF)-dependent quorum sensing (QS) system adopts a novel protein-protein interaction mechanism to autoregulate the production of signal DSF. Here, we present the crystal structures of DSF synthase RpfF and its complex with the REC domain of sensor protein RpfC. RpfF is structurally similarity to the members of the crotonase superfamily and contains an N-terminal α/β spiral core domain and a C-terminal α-helical region. Further structural and mutational analysis identified two catalytic glutamate residues, which is the conserved feature of the enoyl-CoA hydratases/dehydratases. A putative substrate-binding pocket was unveiled and the key roles of the residues implicated in substrate binding were verified by mutational analysis. The binding of the REC domain may lock RpfF in an inactive conformation by blocking the entrance of substrate binding pocket, thereby negatively regulating DSF production. These findings provide a structural model for the RpfC-RpfF interaction-mediated QS autoinduction mechanism.
Organizational Affiliation:
Institute of Molecular and Cell Biology, 61 Biopolis Drive, Proteos, Singapore.