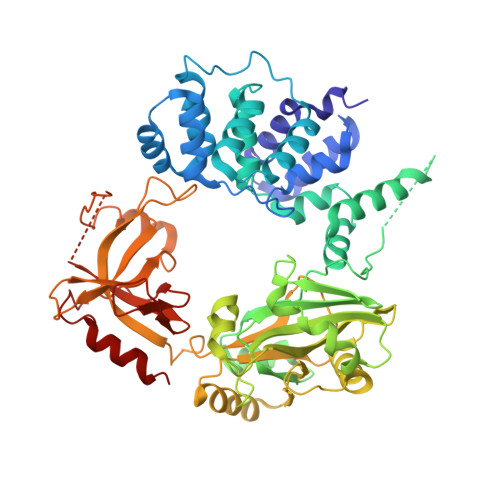
Human DNA Ligase III Recognizes DNA Ends by Dynamic Switching between Two DNA-Bound States.
Cotner-Gohara, E., Kim, I.K., Hammel, M., Tainer, J.A., Tomkinson, A.E., Ellenberger, T.(2010) Biochemistry 49: 6165-6176
- PubMed: 20518483
- DOI: https://doi.org/10.1021/bi100503w
- Primary Citation of Related Structures:
3L2P - PubMed Abstract:
Human DNA ligase III has essential functions in nuclear and mitochondrial DNA replication and repair and contains a PARP-like zinc finger (ZnF) that increases the extent of DNA nick joining and intermolecular DNA ligation, yet the bases for ligase III specificity and structural variation among human ligases are not understood. Here combined crystal structure and small-angle X-ray scattering results reveal dynamic switching between two nick-binding components of ligase III: the ZnF-DNA binding domain (DBD) forms a crescent-shaped surface used for DNA end recognition which switches to a ring formed by the nucleotidyl transferase (NTase) and OB-fold (OBD) domains for catalysis. Structural and mutational analyses indicate that high flexibility and distinct DNA binding domain features in ligase III assist both nick sensing and the transition from nick sensing by the ZnF to nick joining by the catalytic core. The collective results support a "jackknife model" in which the ZnF loads ligase III onto nicked DNA and conformational changes deliver DNA into the active site. This work has implications for the biological specificity of DNA ligases and functions of PARP-like zinc fingers.
Organizational Affiliation:
Department of Biochemistry and Molecular Biophysics, Washington University School of Medicine, St. Louis, Missouri 63110, USA.