The crystal structure of a functionally unknown conserved protein from Enterococcus faecalis V583
Tan, K., Rakowski, E., Jedrzejczak, R., Joachimiak, A.To be published.
Experimental Data Snapshot
wwPDB Validation 3D Report Full Report
Entity ID: 1 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Uncharacterized protein | 257 | Enterococcus faecalis | Mutation(s): 0 Gene Names: EF_1237 | 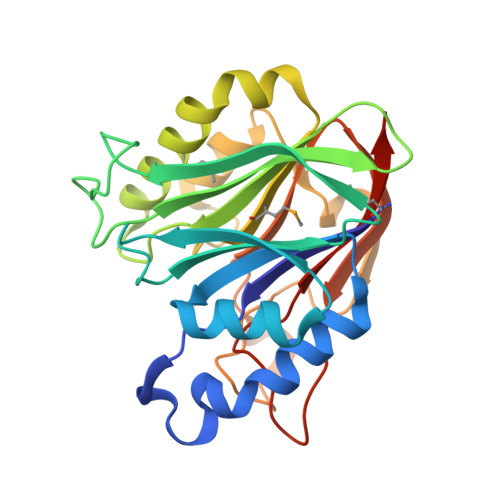 | |
UniProt | |||||
Find proteins for Q835Y1 (Enterococcus faecalis (strain ATCC 700802 / V583)) Explore Q835Y1 Go to UniProtKB: Q835Y1 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | Q835Y1 | ||||
Sequence AnnotationsExpand | |||||
| |||||
| Ligands 3 Unique | |||||
|---|---|---|---|---|---|
| ID | Chains | Name / Formula / InChI Key | 2D Diagram | 3D Interactions | |
| SO4 Query on SO4 | BA [auth D] CA [auth D] DA [auth D] EA [auth D] G [auth A] | SULFATE ION O4 S QAOWNCQODCNURD-UHFFFAOYSA-L |  | ||
| EDO Query on EDO | FA [auth D] K [auth A] MA [auth E] R [auth B] TA [auth F] | 1,2-ETHANEDIOL C2 H6 O2 LYCAIKOWRPUZTN-UHFFFAOYSA-N |  | ||
| FMT Query on FMT | AA [auth C] GA [auth D] HA [auth D] L [auth A] M [auth A] | FORMIC ACID C H2 O2 BDAGIHXWWSANSR-UHFFFAOYSA-N |  | ||
| Modified Residues 1 Unique | |||||
|---|---|---|---|---|---|
| ID | Chains | Type | Formula | 2D Diagram | Parent |
| MSE Query on MSE | A, B, C, D, E A, B, C, D, E, F | L-PEPTIDE LINKING | C5 H11 N O2 Se |  | MET |
| Length ( Å ) | Angle ( ˚ ) |
|---|---|
| a = 68.565 | α = 89.88 |
| b = 68.558 | β = 89.79 |
| c = 104.245 | γ = 60.07 |
| Software Name | Purpose |
|---|---|
| SBC-Collect | data collection |
| SHELXD | phasing |
| MLPHARE | phasing |
| DM | model building |
| ARP | model building |
| WARP | model building |
| HKL-3000 | phasing |
| PHENIX | refinement |
| HKL-3000 | data reduction |
| HKL-3000 | data scaling |
| DM | phasing |