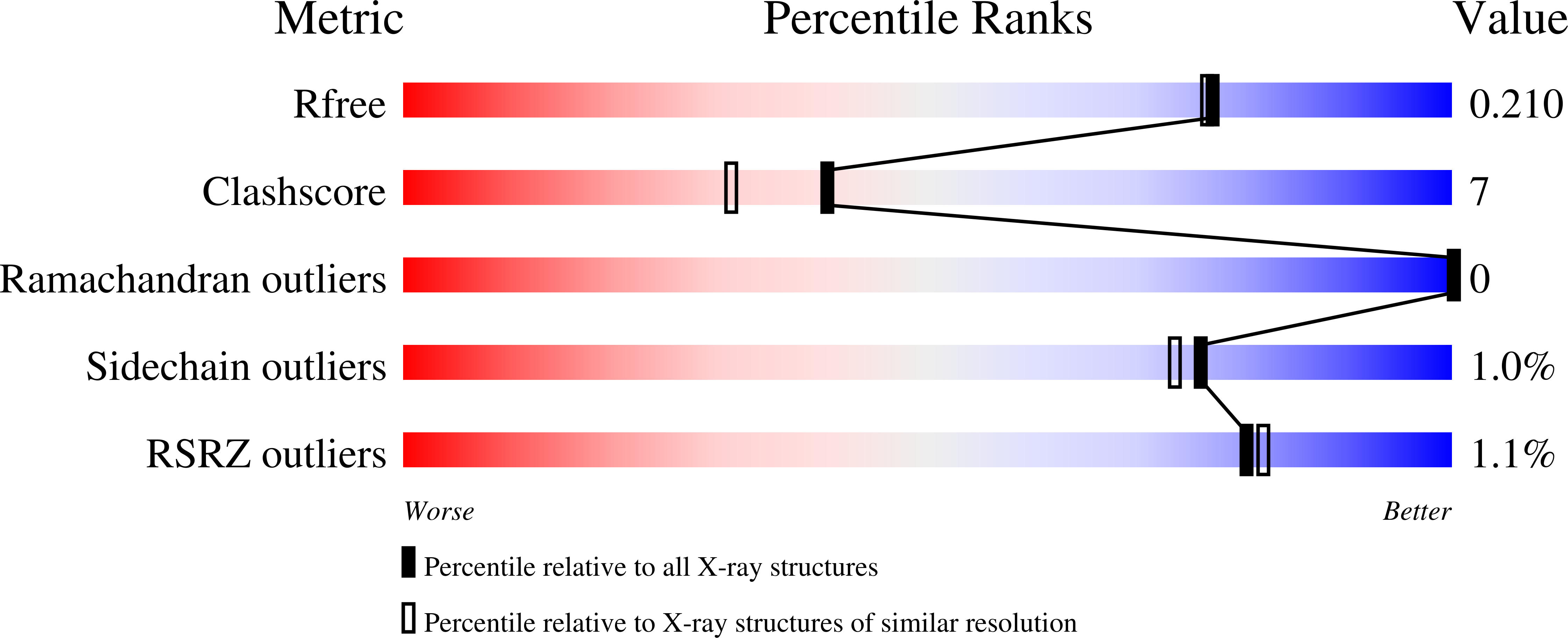
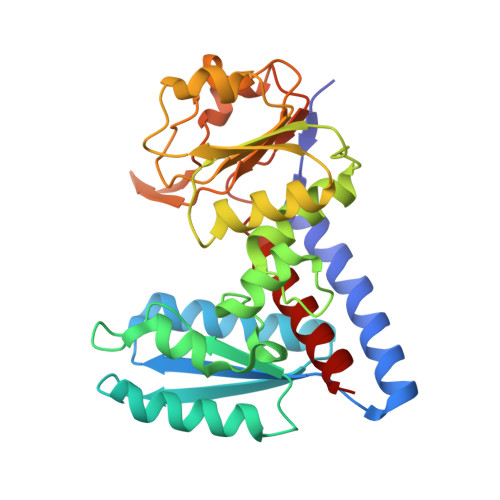
X-ray crystal structure of methylenetetrahydrofolate dehydrogenase/methenyltetrahydrofolate cyclohydrolase, putative bifunctional protein folD from Francisella tularensis.
Osipiuk, J., Maltseva, N., Mulligan, R., Hasseman, J., Anderson, W.F., Joachimiak, A.To be published.