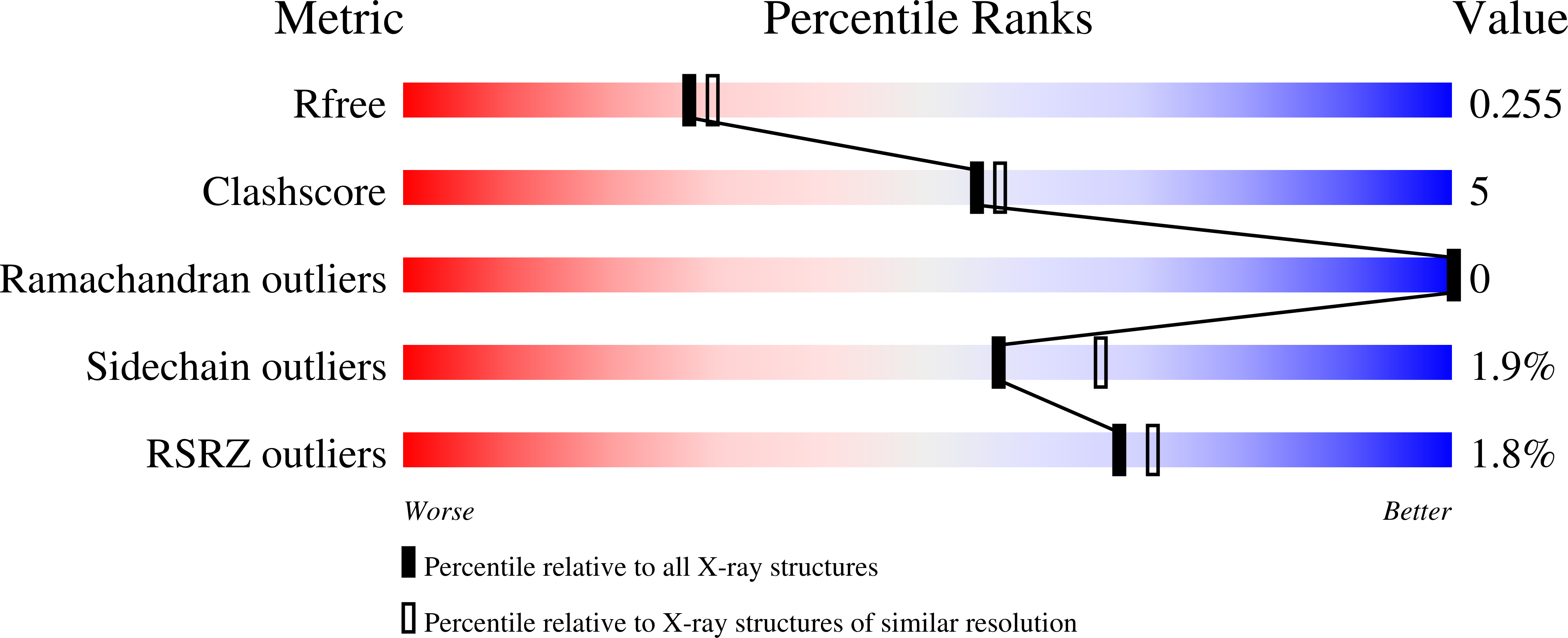
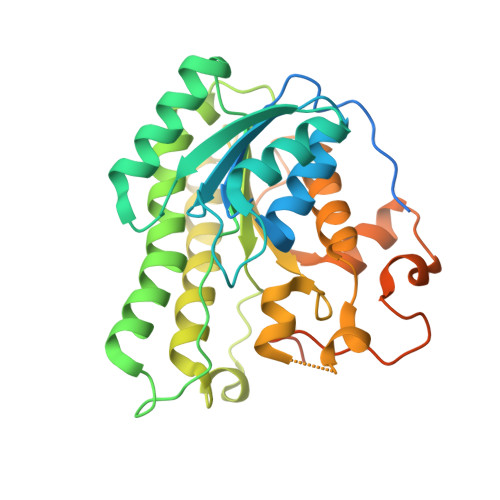
Crystal structure of the catalytic domain of human Hydroxysteroid dehydrogenase like 2 (HSDL2)
Ugochukwu, E., Bhatia, C., Huang, J., Pilka, E., Muniz, J.R.C., Pike, A.C.W., Krojer, T., von Delft, F., Verdin, E.M., Oppermann, U., Kavanagh, K.L.To be published.