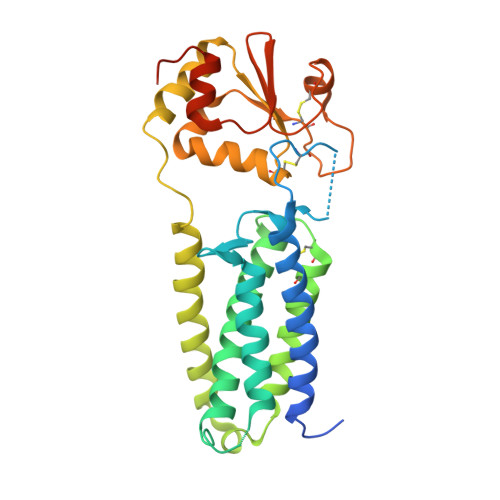
Structure of a bacterial homologue of vitamin K epoxide reductase.
Li, W., Schulman, S., Dutton, R.J., Boyd, D., Beckwith, J., Rapoport, T.A.(2010) Nature 463: 507-512
- PubMed: 20110994
- DOI: https://doi.org/10.1038/nature08720
- Primary Citation of Related Structures:
3KP8, 3KP9 - PubMed Abstract:
Vitamin K epoxide reductase (VKOR) generates vitamin K hydroquinone to sustain gamma-carboxylation of many blood coagulation factors. Here, we report the 3.6 A crystal structure of a bacterial homologue of VKOR from Synechococcus sp. The structure shows VKOR in complex with its naturally fused redox partner, a thioredoxin-like domain, and corresponds to an arrested state of electron transfer. The catalytic core of VKOR is a four transmembrane helix bundle that surrounds a quinone, connected through an additional transmembrane segment with the periplasmic thioredoxin-like domain. We propose a pathway for how VKOR uses electrons from cysteines of newly synthesized proteins to reduce a quinone, a mechanism confirmed by in vitro reconstitution of vitamin K-dependent disulphide bridge formation. Our results have implications for the mechanism of the mammalian VKOR and explain how mutations can cause resistance to the VKOR inhibitor warfarin, the most commonly used oral anticoagulant.
Organizational Affiliation:
Howard Hughes Medical Institute and Department of Cell Biology, Harvard Medical School, 240 Longwood Avenue, Boston, Massachusetts 02115, USA. weikai@crystal.harvard.edu