The crystal structure of cyclohexadienyl dehydratase precursor from Pseudomonas aeruginosa PA01
Tan, K., Marshall, N., Buck, K., Joachimiak, A.To be published.
Experimental Data Snapshot
wwPDB Validation 3D Report Full Report
Entity ID: 1 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Cyclohexadienyl dehydratase | 239 | Pseudomonas aeruginosa | Mutation(s): 0 Gene Names: PA3475, pheC, Pseudomonas aeruginosa EC: 4.2.1.51 (PDB Primary Data), 4.2.1.91 (PDB Primary Data) | 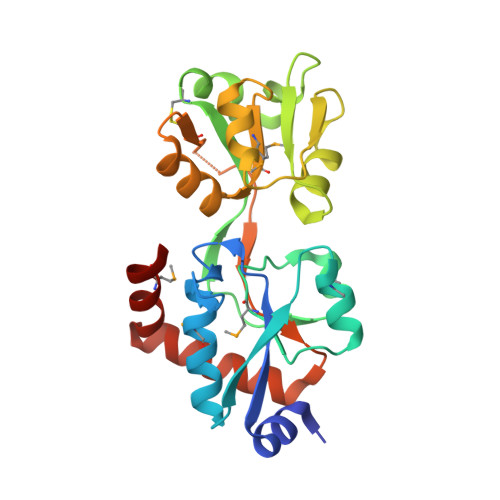 | |
UniProt | |||||
Find proteins for Q01269 (Pseudomonas aeruginosa (strain ATCC 15692 / DSM 22644 / CIP 104116 / JCM 14847 / LMG 12228 / 1C / PRS 101 / PAO1)) Explore Q01269 Go to UniProtKB: Q01269 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | Q01269 | ||||
Sequence AnnotationsExpand | |||||
| |||||
| Ligands 5 Unique | |||||
|---|---|---|---|---|---|
| ID | Chains | Name / Formula / InChI Key | 2D Diagram | 3D Interactions | |
| EPE Query on EPE | K [auth A] | 4-(2-HYDROXYETHYL)-1-PIPERAZINE ETHANESULFONIC ACID C8 H18 N2 O4 S JKMHFZQWWAIEOD-UHFFFAOYSA-N |  | ||
| GOL Query on GOL | D [auth A], E [auth A], F [auth A], H [auth A], I [auth A] | GLYCEROL C3 H8 O3 PEDCQBHIVMGVHV-UHFFFAOYSA-N |  | ||
| NI Query on NI | B [auth A] | NICKEL (II) ION Ni VEQPNABPJHWNSG-UHFFFAOYSA-N |  | ||
| FMT Query on FMT | G [auth A], J [auth A] | FORMIC ACID C H2 O2 BDAGIHXWWSANSR-UHFFFAOYSA-N |  | ||
| CL Query on CL | C [auth A] | CHLORIDE ION Cl VEXZGXHMUGYJMC-UHFFFAOYSA-M |  | ||
| Modified Residues 1 Unique | |||||
|---|---|---|---|---|---|
| ID | Chains | Type | Formula | 2D Diagram | Parent |
| MSE Query on MSE | A | L-PEPTIDE LINKING | C5 H11 N O2 Se |  | MET |
| Length ( Å ) | Angle ( ˚ ) |
|---|---|
| a = 126.39 | α = 90 |
| b = 126.39 | β = 90 |
| c = 88.981 | γ = 120 |
| Software Name | Purpose |
|---|---|
| SBC-Collect | data collection |
| SHELXD | phasing |
| MLPHARE | phasing |
| DM | model building |
| ARP | model building |
| WARP | model building |
| HKL-3000 | phasing |
| PHENIX | refinement |
| HKL-3000 | data reduction |
| HKL-3000 | data scaling |
| DM | phasing |