ErbB2 resembles an autoinhibited invertebrate epidermal growth factor receptor.
Alvarado, D., Klein, D.E., Lemmon, M.A.(2009) Nature 461: 287-291
- PubMed: 19718021
- DOI: https://doi.org/10.1038/nature08297
- Primary Citation of Related Structures:
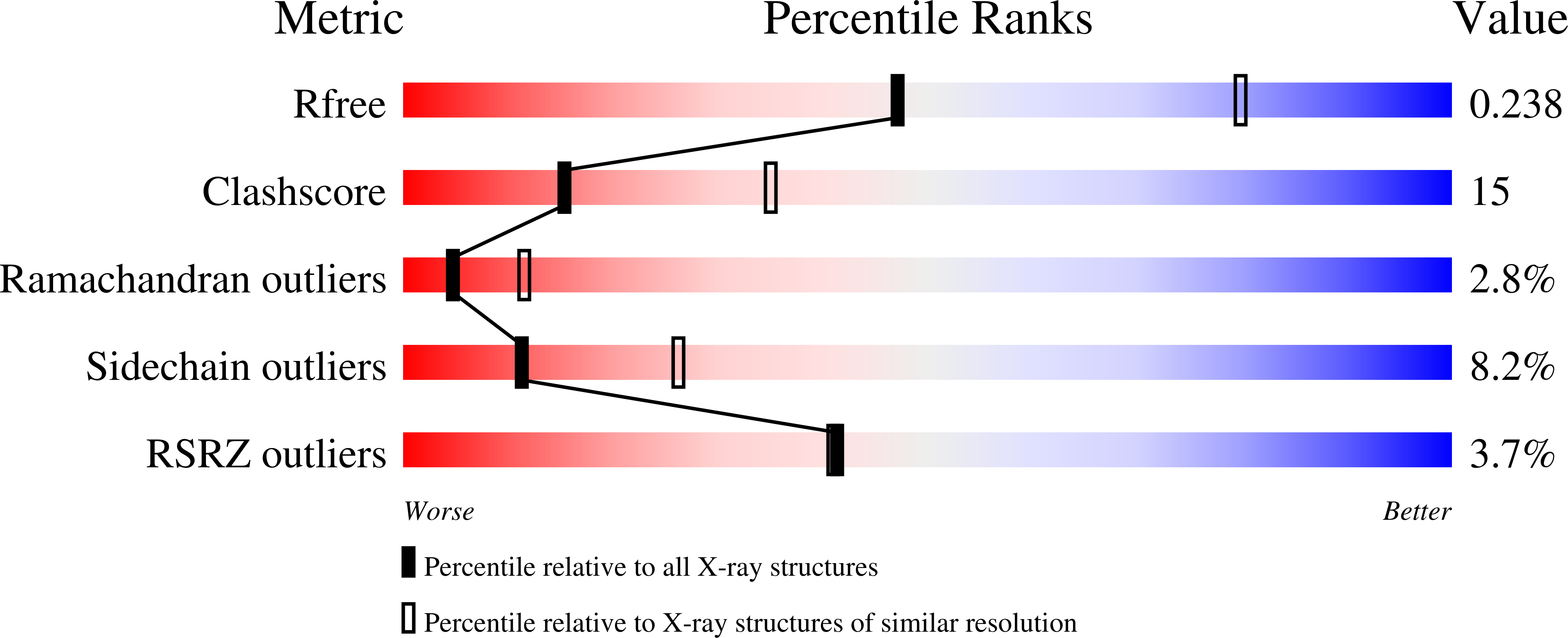
3I2T - PubMed Abstract:
The orphan receptor tyrosine kinase ErbB2 (also known as HER2 or Neu) transforms cells when overexpressed, and it is an important therapeutic target in human cancer. Structural studies have suggested that the oncogenic (and ligand-independent) signalling properties of ErbB2 result from the absence of a key intramolecular 'tether' in the extracellular region that autoinhibits other human ErbB receptors, including the epidermal growth factor (EGF) receptor. Although ErbB2 is unique among the four human ErbB receptors, here we show that it is the closest structural relative of the single EGF receptor family member in Drosophila melanogaster (dEGFR). Genetic and biochemical data show that dEGFR is tightly regulated by growth factor ligands, yet a crystal structure shows that it, too, lacks the intramolecular tether seen in human EGFR, ErbB3 and ErbB4. Instead, a distinct set of autoinhibitory interdomain interactions hold unliganded dEGFR in an inactive state. All of these interactions are maintained (and even extended) in ErbB2, arguing against the suggestion that ErbB2 lacks autoinhibition. We therefore suggest that normal and pathogenic ErbB2 signalling may be regulated by ligands in the same way as dEGFR. Our findings have important implications for ErbB2 regulation in human cancer, and for developing therapeutic approaches that target novel aspects of this orphan receptor.
Organizational Affiliation:
Department of Biochemistry and Biophysics, University of Pennsylvania School of Medicine, 809C Stellar-Chance Laboratories, 422 Curie Boulevard, Philadelphia, Pennsylvania 19104-6059, USA.