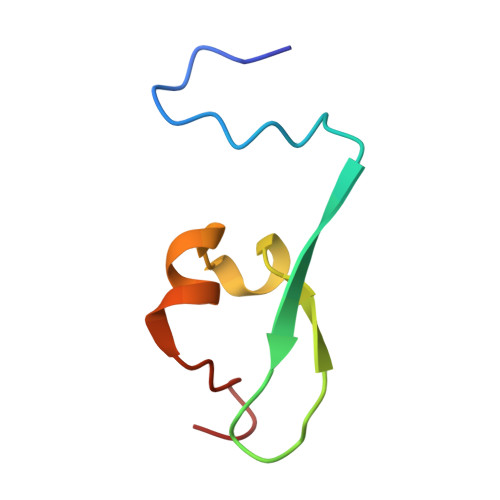
Haematopoietic malignancies caused by dysregulation of a chromatin-binding PHD finger.
Wang, G.G., Song, J., Wang, Z., Dormann, H.L., Casadio, F., Li, H., Luo, J.L., Patel, D.J., Allis, C.D.(2009) Nature 459: 847-851
- PubMed: 19430464
- DOI: https://doi.org/10.1038/nature08036
- Primary Citation of Related Structures:
2KGG, 2KGI, 3GL6 - PubMed Abstract:
Histone H3 lysine 4 methylation (H3K4me) has been proposed as a critical component in regulating gene expression, epigenetic states, and cellular identities1. The biological meaning of H3K4me is interpreted by conserved modules including plant homeodomain (PHD) fingers that recognize varied H3K4me states. The dysregulation of PHD fingers has been implicated in several human diseases, including cancers and immune or neurological disorders. Here we report that fusing an H3K4-trimethylation (H3K4me3)-binding PHD finger, such as the carboxy-terminal PHD finger of PHF23 or JARID1A (also known as KDM5A or RBBP2), to a common fusion partner nucleoporin-98 (NUP98) as identified in human leukaemias, generated potent oncoproteins that arrested haematopoietic differentiation and induced acute myeloid leukaemia in murine models. In these processes, a PHD finger that specifically recognizes H3K4me3/2 marks was essential for leukaemogenesis. Mutations in PHD fingers that abrogated H3K4me3 binding also abolished leukaemic transformation. NUP98-PHD fusion prevented the differentiation-associated removal of H3K4me3 at many loci encoding lineage-specific transcription factors (Hox(s), Gata3, Meis1, Eya1 and Pbx1), and enforced their active gene transcription in murine haematopoietic stem/progenitor cells. Mechanistically, NUP98-PHD fusions act as 'chromatin boundary factors', dominating over polycomb-mediated gene silencing to 'lock' developmentally critical loci into an active chromatin state (H3K4me3 with induced histone acetylation), a state that defined leukaemia stem cells. Collectively, our studies represent, to our knowledge, the first report that deregulation of the PHD finger, an 'effector' of specific histone modification, perturbs the epigenetic dynamics on developmentally critical loci, catastrophizes cellular fate decision-making, and even causes oncogenesis during mammalian development.
Organizational Affiliation:
Laboratory of Chromatin Biology & Epigenetics, The Rockefeller University, New York, New York 10065, USA.