Structural and functional insights into the ligand-binding domain of a nonduplicated retinoid X nuclear receptor from the invertebrate chordate amphioxus
Tocchini-Valentini, G.D., Rochel, N., Escriva, H., Germain, P., Peluso-Iltis, C., Paris, M., Sanglier-Cianferani, S., Van Dorsselaer, A., Moras, D., Laudet, V.(2009) J Biol Chem 284: 1938-1948
- PubMed: 18986992
- DOI: https://doi.org/10.1074/jbc.M805692200
- Primary Citation of Related Structures:
3EYB - PubMed Abstract:
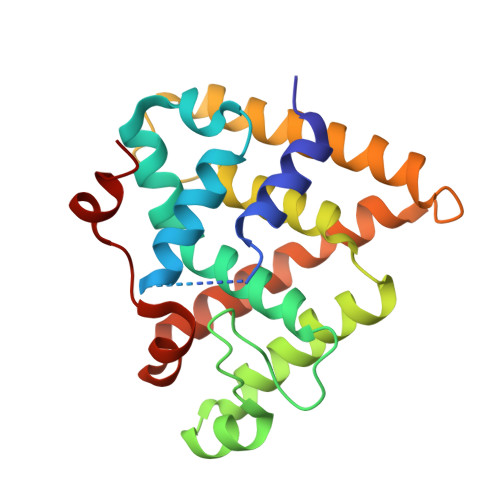
Retinoid X nuclear receptors (RXRs), as well as their insect orthologue, ultraspiracle protein (USP), play an important role in the transcription regulation mediated by the nuclear receptors as the common partner of many other nuclear receptors. Phylogenetic and structural studies have shown that the several evolutionary shifts have modified the ligand binding ability of RXRs. To understand the vertebrate-specific character of RXRs, we have studied the RXR ligand-binding domain of the cephalochordate amphioxus (Branchiostoma floridae), an invertebrate chordate that predates the genome duplication that produced the three vertebrates RXRs (alpha, beta, and gamma). Here we report the crystal structure of a novel apotetramer conformation of the AmphiRXR ligand-binding domain, which shows some similarity with the structures of the arthropods RXR/USPs. AmphiRXR adopts an apo antagonist conformation with a peculiar conformation of helix H11 filling the binding pocket. In contrast to the arthropods RXR/USPs, which cannot be activated by any RXR ligands, our functional data show that AmphiRXR, like the vertebrates/mollusk RXRs, is able to bind and be activated by RXR ligands but less efficiently than vertebrate RXRs. Our data suggest that amphioxus RXR is, functionally, an intermediate between arthropods RXR/USPs and vertebrate RXRs.
Organizational Affiliation:
Département de Biologie et de Génomique Structurales and Département Biologie du Cancer, Institut de Génétique et de Biologie Moléculaire et Cellulaire, UMR7104 CNRS, U596 INSERM, Université Louis Pasteur, 67400 Illkirch, France.