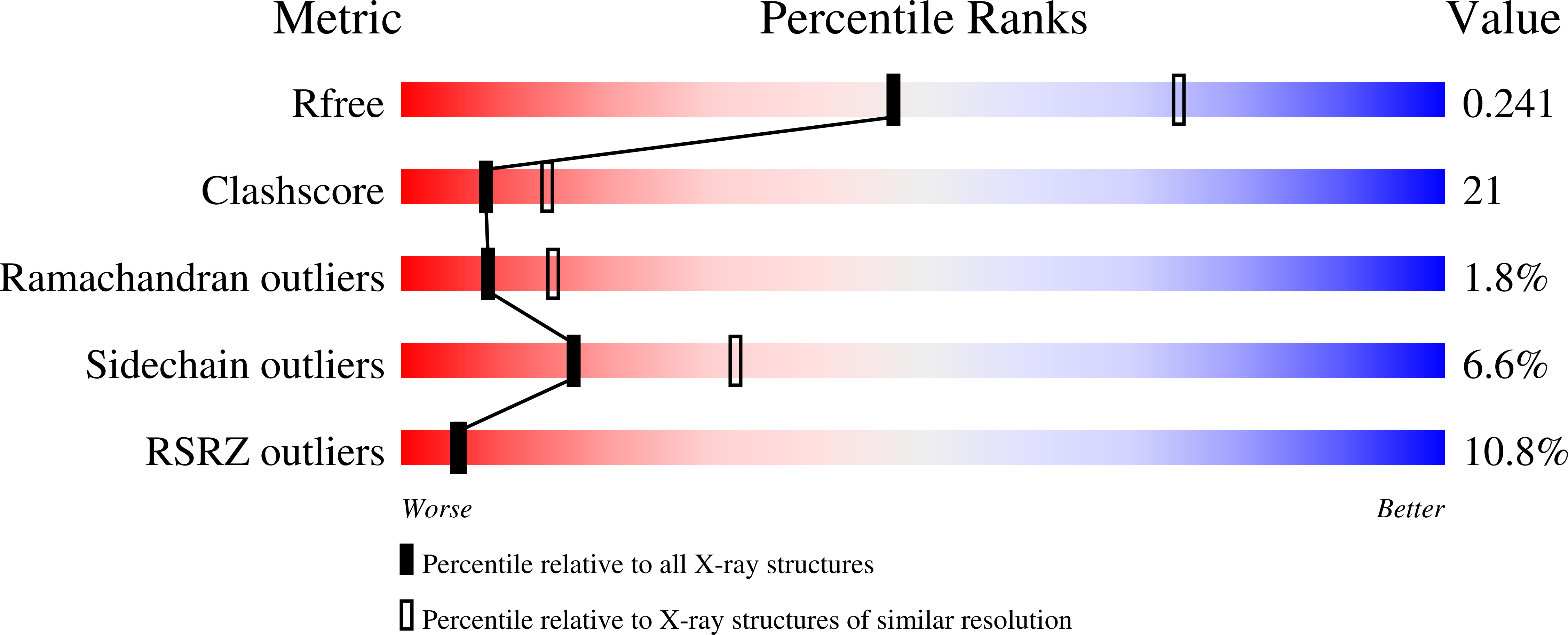
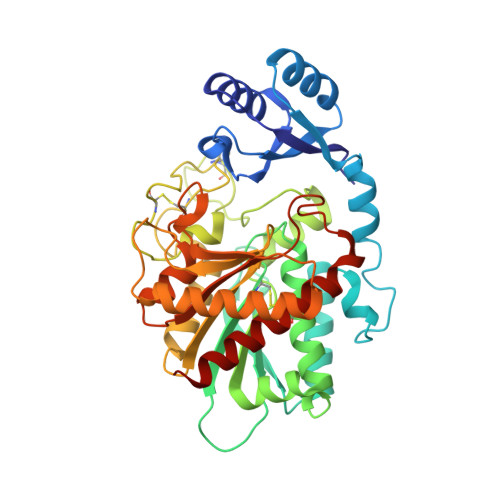
The crystal structure of thrombin-activable fibrinolysis inhibitor (TAFI) provides the structural basis for its intrinsic activity and the short half-life of TAFIa.
Anand, K., Pallares, I., Valnickova, Z., Christensen, T., Vendrell, J., Wendt, K.U., Schreuder, H.A., Enghild, J.J., Aviles, F.X.(2008) J Biol Chem 283: 29416-29423
- PubMed: 18669641
- DOI: https://doi.org/10.1074/jbc.M804003200
- Primary Citation of Related Structures:
3DGV - PubMed Abstract:
Mature thrombin-activable fibrinolysis inhibitor (TAFIa) is a highly unstable metallocarboxypeptidase that stabilizes blood clots by clipping C-terminal lysine residues from partially degraded fibrin. In accordance with its in vitro antifibrinolytic activity, animal studies have reported that inhibition of mature TAFI aids in the prevention of thrombosis. The level of TAFI activity is stringently regulated through (i) controlled proteolytic truncation of the zymogen (TAFI), generating the mature enzyme, TAFIa, and (ii) the short half-life of TAFIa. TAFI itself exhibits an intrinsic enzymatic activity, which is likely required to provide a baseline level of antifibrinolytic activity. The novel crystal structure presented here reveals that the active site of TAFI is accessible, providing the structural explanation for the its intrinsic activity. It also supports the notion that an "instability region" exists, in agreement with site-directed mutagenesis studies. Sulfate ions, bound to this region, point toward a potential heparin-binding site and could explain how heparin stabilizes TAFIa.
Organizational Affiliation:
Sanofi-Aventis Pharma Deutschland GmbH, Industriepark Höchst, 65926 Frankfurt am Main, Germany.