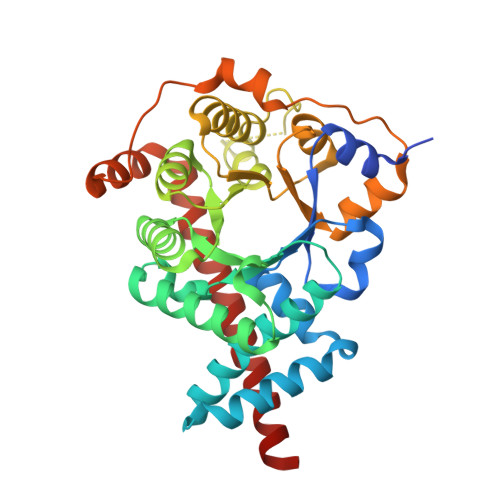
The crystal structure and identification of NQM1/YGR043C, a transaldolase from Saccharomyces cerevisiae
Huang, H., Rong, H., Li, X., Tong, S., Zhu, Z., Niu, L., Teng, M.(2008) Proteins 73: 1076-1081
- PubMed: 18831051
- DOI: https://doi.org/10.1002/prot.22237
- Primary Citation of Related Structures:
3CQ0
Organizational Affiliation:
School of Life Sciences, Hefei National Laboratory for Physical Sciences at Microscale, University of Science and Technology of China, Hefei, Anhui 230026, People's Republic of China.